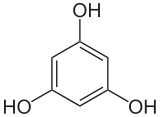
1,3,5-trihydroxybenzene
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | 1,3,5-trihydroxybenzene | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 6 H 6 O 3 | |||||||||||||||||||||
| Brief description |
colorless to light beige solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 126.11 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
215-218 ° C |
|||||||||||||||||||||
| pK s value |
8.45 |
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
1,3,5-Trihydroxybenzene (common name phloroglucinol ) is a derivative of benzene , a trihydric phenol . It crystallizes from aqueous solution as a dihydrate with two molecules of crystal water . Hydrochloric acid 1,3,5-trihydroxybenzene solution serves as a detection reagent for lignin . The other two isomers are 1,2,3-trihydroxybenzene (pyrogallol) and 1,2,4-trihydroxybenzene (hydroxyhydroquinone).
presentation
1,3,5-Trihydroxybenzene can be obtained from aqueous solutions in the form of colorless crystal needles, which contain 2 molecules of crystal water per molecule of 1,3,5-trihydroxybenzene: C 6 H 3 (OH) 3 · 2 H 2 O. Es can be obtained by splitting many plant substances with a higher composition, such as dragon blood , gummy tart , quercetin , morin or maclurin.
properties
Physical Properties
The 1,3,5-trihydroxybenzene, which is free of water of crystallization, has a melting point of approx. 218 ° C .; it decomposes before the boiling point is reached. It dissolves easily in alcohol and ether and tastes very sweet.
Chemical properties
Many natural substances such as flavones and anthocyanin dyes are derived from 1,3,5-trihydroxybenzene . Chemically, it often does not behave like a phenol , but like a ketone . It forms a trioxime with hydroxylamine.
Formally, it can therefore also be understood as a (cyclo) trimer of the ketene (H 2 C = C = O). 1,3,5-trihydroxybenzene is light-sensitive and is therefore stored in brown glass bottles.
use
Hydrochloric acid 1,3,5-trihydroxybenzene solution is used in paper production to detect lignin contained in wood pulp . If lignin is present in a sample, it will turn red. The carbonyl group of the lignin contained in the reacted Coniferylaldehyds with the 1,3,5-trihydroxybenzene. It is also part of Günzburg's reagent - an alcoholic solution of 1,3,5-trihydroxybenzene and vanillin for the qualitative detection of free hydrochloric acid in gastric juice .
In microscopy it is used to decalcify bone samples. 1,3,5-trihydroxybenzene also serves as a reagent for pentoses , pentosans and aldehydes .
proof
For qualitative analytical evidence, bromination with potassium bromide and bromine produces tribromophloroglucine , which has a melting point of 151 ° C.
See also
Individual evidence
- ↑ a b c d data sheet 1,3,5-trihydroxybenzene from Acros, accessed on November 27, 2014.
- ↑ CRC Handbook of Tables for Organic Compound Identification , Third Edition, 1984, ISBN 0-8493-0303-6 .
- ↑ a b entry on phloroglucinol. In: Römpp Online . Georg Thieme Verlag, accessed on September 24, 2014.
- ↑ a b Entry on phloroglucine in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ^ A b Meyers Konversations-Lexikon, 1888 .
- ↑ Experimental lecture "From the tree to the apple", Marietta Fischer, University of Marburg ( MS Word ; 2.1 MB).
- ^ Association of authors: Organikum , 19th edition, Johann Ambrosius Barth, Leipzig · Berlin · Heidelberg 1993, ISBN 3-335-00343-8 , p. 331.
- ^ Association of authors: Organikum , 19th edition, Johann Ambrosius Barth, Leipzig · Berlin · Heidelberg 1993, ISBN 3-335-00343-8 , p. 653.



