Morin (dye)
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
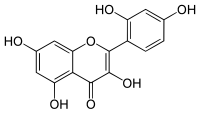
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Morin | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 15 H 10 O 7 | ||||||||||||||||||
| Brief description |
yellow powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 302.24 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.799 g cm 3 (20 ° C) |
||||||||||||||||||
| Melting point |
285–290 ° C (decomposition, Morin) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Morin or 2 ', 3,4', 5,7-pentahydroxy flavone is contained in maclurin (2,3 ', 4,4', 6-pentahydroxybenzophenone) in the yellow wood of the dyer's mulberry tree ( Morus tinctoria ), its extract for wool dyeing and the cotton print is important. The colors are yellow. Morin crystallizes as a morin hydrate with a molecule or two of water. Morin serves as a reagent on aluminum which fluoresces green in neutral and acetic acidic alcoholic solution . Beryllium , on the other hand, produces the same fluorescence in an alkaline solution, so that both metals can be detected side by side. Also, gallium , indium , scandium , titanium , and tin can be detected by Morin. Morin was also used for development dyeing of cotton, leather, silk, and wool. For this purpose, the goods to be dyed were first pretreated with metal salts; different colorations could be achieved by reaction of the morine with the metal ions. Morin, for example, produces olive-green coloring with iron salts, brownish-yellow with copper salts , and yellow lacquers with lead and tin salts .
literature
- Paul Karrer: Textbook of Organic Chemistry , 10th edition 1948, Georg Thieme Verlag Stuttgart, p. 589
Individual evidence
- ↑ a b Data sheet Morin hydrate from Sigma-Aldrich , accessed on April 11, 2011 ( PDF ).
- ↑ a b c d Entry on Morin in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ^ The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals , 14th Edition (Merck & Co., Inc.), Whitehouse Station, NJ, USA, 2006; P. 1082, ISBN 978-0-911910-00-1 .
- ↑ a b c d e entry on Morin. In: Römpp Online . Georg Thieme Verlag, accessed on May 3, 2019.

