beryllium
| properties | |||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General | |||||||||||||||||||||||||||||||||||||
| Name , symbol , atomic number | Beryllium, Be, 4 | ||||||||||||||||||||||||||||||||||||
| Element category | Alkaline earth metals | ||||||||||||||||||||||||||||||||||||
| Group , period , block | 2 , 2 , s | ||||||||||||||||||||||||||||||||||||
| Appearance | white-gray metallic | ||||||||||||||||||||||||||||||||||||
| CAS number | 7440-41-7 | ||||||||||||||||||||||||||||||||||||
| EC number | 231-150-7 | ||||||||||||||||||||||||||||||||||||
| ECHA InfoCard | 100.028.318 | ||||||||||||||||||||||||||||||||||||
| Mass fraction of the earth's envelope | 5.3 ppm | ||||||||||||||||||||||||||||||||||||
| Atomic | |||||||||||||||||||||||||||||||||||||
| Atomic mass | 9.0121831 (5) et al | ||||||||||||||||||||||||||||||||||||
| Atomic radius (calculated) | 105 (112) pm | ||||||||||||||||||||||||||||||||||||
| Covalent radius | 96 pm | ||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 153 pm | ||||||||||||||||||||||||||||||||||||
| Electron configuration | [ He ] 2 s 2 | ||||||||||||||||||||||||||||||||||||
| 1. Ionization energy | 9.322 699 (7) eV ≈ 899.5 kJ / mol | ||||||||||||||||||||||||||||||||||||
| 2. Ionization energy | 18th.21115 (4) eV ≈ 1 757.11 kJ / mol | ||||||||||||||||||||||||||||||||||||
| 3. Ionization energy | 153.896 203 (4) eV ≈ 14 848.72 kJ / mol | ||||||||||||||||||||||||||||||||||||
| 4. Ionization energy | 217.718 584 3 (17) eV ≈ 21 006.64 kJ / mol | ||||||||||||||||||||||||||||||||||||
| Physically | |||||||||||||||||||||||||||||||||||||
| Physical state | firmly | ||||||||||||||||||||||||||||||||||||
| Crystal structure | hexagonal (closest packed) | ||||||||||||||||||||||||||||||||||||
| density | 1.848 g / cm 3 (20 ° C ) | ||||||||||||||||||||||||||||||||||||
| Mohs hardness | 5.5 | ||||||||||||||||||||||||||||||||||||
| magnetism | diamagnetic ( Χ m = −2.3 10 −5 ) | ||||||||||||||||||||||||||||||||||||
| Melting point | 1560 K (1287 ° C) | ||||||||||||||||||||||||||||||||||||
| boiling point | 3243 K (2969 ° C) | ||||||||||||||||||||||||||||||||||||
| Molar volume | 4.85 · 10 −6 m 3 · mol −1 | ||||||||||||||||||||||||||||||||||||
| Heat of evaporation | 309 kJ / mol | ||||||||||||||||||||||||||||||||||||
| Heat of fusion | 7.95 kJ mol −1 | ||||||||||||||||||||||||||||||||||||
| Speed of sound | 13000 m s −1 | ||||||||||||||||||||||||||||||||||||
| Specific heat capacity | 1825 J kg −1 K −1 | ||||||||||||||||||||||||||||||||||||
| Work function | 4.98 eV | ||||||||||||||||||||||||||||||||||||
| Electric conductivity | 25 · 10 6 A · V −1 · m −1 | ||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 190 W m −1 K −1 | ||||||||||||||||||||||||||||||||||||
| Chemically | |||||||||||||||||||||||||||||||||||||
| Oxidation states | 2 | ||||||||||||||||||||||||||||||||||||
| Normal potential | −1.97 V (Be 2+ + 2 e - → Be) | ||||||||||||||||||||||||||||||||||||
| Electronegativity | 1.57 ( Pauling scale ) | ||||||||||||||||||||||||||||||||||||
| Isotopes | |||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
| For other isotopes see list of isotopes | |||||||||||||||||||||||||||||||||||||
| NMR properties | |||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
| safety instructions | |||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
| Toxicological data | |||||||||||||||||||||||||||||||||||||
|
As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . |
|||||||||||||||||||||||||||||||||||||
Beryllium is a chemical element with the symbol Be and the atomic number 4. Its name is derived from the mineral beryl , a gemstone containing beryllium ( ancient Greek βήρυλλος bēryllos , German 'sea-green gem, beryl' , Latin beryllos , sea-green Indian gem, beryl ' ) . Most of the beryllium in the earth's crust is bound in this mineral and in bertrandite . Beryllium is one of the less common metals .
It was discovered as a component of the mineral beryl by Louis-Nicolas Vauquelin as early as 1798 and was initially called glucine because of the sweet taste of the isolated beryllium-containing compounds (e.g. hydroxide ) . Elemental beryllium was first produced in 1828 by Friedrich Wöhler and independently of that by Antoine Bussy .
In the periodic table , beryllium is in the second main group (2nd IUPAC group ) and is therefore one of the alkaline earth metals . As an element of the second period, it is one of the light alkaline earth metals. However, due to the relationship between the charge and the diameter of the divalent ion, it exhibits some unusual properties for this group, such as a higher density than its two homologues magnesium and calcium .
The steel-gray light metal is very hard and brittle, has a higher modulus of elasticity than steel and is mostly used as an alloy additive. In compounds it is bivalent . Compared to the other light alkali and alkaline earth metals ( lithium , sodium , potassium , magnesium and calcium), it is exceptionally toxic and harmful.
history
In ancient times and in the Middle Ages, pieces of transparent beryl were often used as visual aids, magnifying the writing and images like a magnifying glass when reading.
The first recorded mention is found in the Naturalis historia from the 1st century, where Pliny the Elder describes the similarity between the minerals beryl and emerald (beryl with a trace of chrome), but considers them to be different substances.
The Abbé R. J. Haüy determined that beryl and emerald had the same physical properties and the same crystal form in terms of hardness and density, and prompted the French chemist Louis-Nicolas Vauquelin to conduct an investigation.

Vauquelin proved in 1798 that beryl and emerald are almost chemically identical. He also isolated an oxide from both minerals, which he called terre du béril (beryl clay); it was similar to the known aluminum compounds, but clearly different from them. Until then, according to the previous analyzes by T. Bergman , FC Achard, M. H. Klaproth and LN Vauquelin , beryl was thought to be a calcium aluminum silicate. The editors of Vauquelin's article named the newly discovered substance glucine because of the sweet taste of the beryllium salt (Greek γλυκύς glykys , 'sweet'). Names such as Glucinum or Glucinium were used in France and some other countries until the 20th century, although MH Klaproth and AG Ekeberg already pointed out in 1802 that sweet taste is by no means a special property of the beryllium salts, yttrium salts also taste sweet , and therefore the designation beryl clay is preferable.
The first reports of attempts to represent the element were published by H. Davy in 1809 and by F. Stromeyer in 1812 . But it was not until 1828 that Friedrich Wöhler and shortly afterwards Antoine Bussy succeeded in producing the element by reducing the beryllium chloride with potassium . Wöhler named the new element beryllium . The element discovered in 1836 in davidsonite (a variety of beryl), called Donium by T. Richardson and Treenium by HS Boase , turned out to be beryllium. M. Avdejew made the first determinations of the exact atomic weight in 1842. Julius Weeren (1854) and Henry Debray (1855) also carried out extensive studies of the metal, its elementary properties and its compounds. Even Charles Arad Joy (1863) explored the production of Beryllverbindungen. It became known to other circles at the Paris World's Fair in 1867, where a large amount was exhibited for the first time.
The chemical symbol Be was introduced by JJ Berzelius in 1814 .
Great advances in beryllium chemistry were made between 1873 and 1885 by Albert Atterberg , LF Nilson and O. Pettersson. During these years the valence of beryllium and its position in the periodic table was also discussed intensively. Numerous other researchers later also contributed to the development of the chemistry of beryllium.
Pure beryllium in crystalline form was first produced in 1899 by Paul Marie Alfred Lebeau by electrolysis of sodium tetrafluoridoberyllate (Na 2 [BeF 4 ]). After the First World War, beryllium was produced simultaneously by Siemens & Halske AG (Alfred Stock and Hans Goldschmidt) in Germany and by Union Carbide and Carbon Corporation (Hugh S. Cooper) in the United States. After the Second World War, the Beryllium Corp. of America in Cleveland produced high-purity beryllium and in England the National Physical Laboratory researched the element.
In 1945 beryllium was used together with the alpha emitter polonium as a neutron source in the Little Boy atomic bomb that was dropped over Hiroshima .
Beryllium compounds were used as a component in fluorescent tubes until the 1940s (e.g. zinc-manganese-beryllium-silicate), but this ended when more and more cases of beryllium disease were uncovered. Beryllium was also first described in 1946 on the basis of workers in this industry.
Occurrence
In the solar system, beryllium is the rarest of the elements that are lighter than iron (see the frequencies listed in the solar system ). In the earth's shell , with a mass fraction of 5.3 ppm, it is in 48th place in terms of the abundance of elements . It is highly enriched in the upper continental lithosphere , comparing the concentrations of 1.4 ppm in the lower continental crust and 0.07 ppm in the primitive mantle.
Beryllium is a typical lithophilic element. It forms a characteristic four-fold coordination with oxygen in the [BeO 4 ] 6− complex. Geochemically, it is enriched in acidic and alkaline magmas during the magmatic differentiation process. In the case of an acidic magma, it is concentrated in the pegmatitic and hydrothermal residual phase, while in the case of an alkaline magma it enters the lattice of several rock-forming and additional minerals by means of a diadochic trapping technique , which prevents its concentration in the residual phase.
Minerals that contain beryllium as an essential component appear to have emerged relatively late. Such have not been detected in terrestrial rocks older than about 3 billion years; they probably only appear around 1.5 billion years after the earth was formed. So far, no beryllium-containing minerals have been found in extraterrestrial rocks. Meteorites such as chondrites , achondrites , stony iron and iron meteorites contain beryllium in concentrations of 0 to 400 ppb. In local calcium-aluminum-rich inclusions (CAIs) up to 560 ppb are achieved, with a maximum concentration in melilite and change phases of CAIs (649 ppb or ≈ 1 ppm); beryllium's affinity for melilite is attributed to its structural similarity to gugiaite . Concentrations below 10 ppm are rarely sufficient to stabilize a mineral with beryllium as an essential component. Normally, a further enrichment is necessary so that the more common beryllium minerals can arise, for example to around 70 ppm for a beryl in granitic pegmatites.
The rare element beryllium occurs in a number of different minerals on earth . The most important in terms of quantity are bertrandite (Be 4 Si 2 O 7 (OH) 2 ) and beryl (Be 3 Al 2 Si 6 O 18 ). Also Phenakit occurs worldwide. Beryllium occurs in the structure of almost 40 minerals as a formula-active component and in a good 50 other minerals as a diadochic component (some sources even give 112, the International Mineralogical Association 126 (as of July 2019) minerals with beryllium as the essential element). Of the approximately 40 actual beryllium minerals, 26 are silicates (e.g. beryl, barylite , phenakite), which show the close geochemical similarity of the complex [BeO 4 ] 6− with the complexes of [SiO 4 ] 4− and [AlO 4 ] 5 - reflect. In addition, there are oxides (e.g. bromellite , chrysoberyl ), borates (e.g. hambergite , rhodicite ), antimonates (e.g. Swedenborgite ), phosphates (e.g. beryllonite , hurlbutite ) and the only known carbonate, niveolanite known. Beryl occurs in heterogeneous zoned pegmatites, bertrandite comes from non-pegmatitic sources.
The most beautiful and most valuable beryllium-containing minerals are among others the beryl varieties aquamarine , emerald and other "beryls", chrysoberyl and its variety alexandrite as well as euclas , phenakite and tugtupite , which are mainly used as gemstones . Beryllium deposits are mainly found in the equatorial belt. Until a few years ago, emerald was extracted in the Leckbachrinne in the Habach Valley (Hohe Tauern) south of Bramberg in Austria (see also the Habachtal Emerald Mine ). In the United States, low-grade beryllium oxide ore deposits are being mined in the Nevada desert. The estimated reserves of recoverable beryllium are around 80,000 tons worldwide. About 65 percent of the deposits are in the United States (mainly in the form of bertrandite in the Spor Mountain area of Utah) and the remainder in beryl deposits in other countries. These are mined in Russia , Canada , Brazil , China , Madagascar , Mozambique and Portugal .
Beryllium occurs in traces in many substances.
| material | Beryllium content |
|---|---|
| coal | 1.8-2.2 mg / kg |
| Coal ash | 46 mg / kg |
| Chimney emissions from coal-fired power plants | 0.8 µg / m 3 |
| Cigarettes | up to 0.74 µg per cigarette |
| fertilizer | <200-13,500 µg / kg |
| US drinking water | 0.5 µg / l |
| Air (US average) | <3 · 10 −5 µg / m 3 |
| Kidney beans | 2200 µg / kg |
| crispbread | 112 µg / kg |
| Garden peas | 109 µg / kg |
Manufacturing
The most important starting material for the preparation of beryllium salts as the starting material for the production of beryllium is beryl, which in addition to the aluminum content given by the formula usually also contains iron . In addition to the actual digestion, the separation of beryllium from aluminum and iron is important. The digestion takes place either with an alkaline flux
or by means of fluorides or silicofluorides.
In addition to beryl, gadolinite and leucophane are also used as starting materials for beryllium salts with digestion by, for example, sulfuric acid or aqua regia .
The resulting beryllium hydroxide is reacted with ammonium bifluoride to form ammonium fluoroberyllate , which in turn is decomposed at elevated temperatures (> 125 ° C) to beryllium fluoride and volatile ammonium fluoride . Beryllium chloride is produced by converting beryllium hydroxide into beryllium oxide by heating , which reacts at 800 ° C. with carbon and chlorine to form the desired anhydrous beryllium chloride.
Elemental beryllium is produced by reducing beryllium fluoride with magnesium at 1300 ° C. The reaction starts at low temperatures, but above 850 ° C the reaction rate increases after both magnesium and beryllium fluoride have melted.
The production of high-purity, metallic beryllium is carried out by fused- salt electrolysis of beryllium chloride with lithium chloride at 500 ° C or sodium chloride at 350 ° C or beryllium fluoride with lithium fluoride or potassium fluoride at 500 ° C:
Beryllium is deposited on the cathode in the form of a fine beryllium powder, which from time to time is lifted from the melt with the cathode and stripped off and - after removing any adhering salt (washing with water) - is transformed into compact pieces by sintering at 1150 ° C becomes.
The annual world production of beryllium metal was around 230 t in 2018 . The worldwide reserves proven to date amount to over 100,000 t. The price of beryllium as a fully machined aerospace component is between 300 and 1500 € / kg. As a commercial product in the form of wires or foils made of pure beryllium (> 99.5% beryllium content) also significantly more expensive (> 10,000 € / g.)
properties
Physical Properties
Crystalline beryllium is steel-gray in color, with well-developed crystal faces often showing a lighter hue. The Mohs hardness of the metal is between 6 and 7. It is diamagnetic and extremely brittle at normal temperatures and cracks easily when subjected to impact. At higher temperatures it is relatively ductile, but machining at these temperatures is very difficult because of the very high affinity of the metal for oxygen and can only be carried out in a hydrogen atmosphere or in a vacuum without loss of material. The brittle behavior depends on various factors such as temperature, grain size, purity (especially the beryllium oxide content) and the processing method. Very fine-grained and highly pure (99.999%) beryllium can be plastically deformed at normal temperatures. Technical grade beryllium can be deformed at temperatures above 500 ° C.
Beryllium has a remarkably high melting point for a light metal . In addition to the very high specific heat capacity of 1.825 kJ / (kg · K), it has a modulus of elasticity that is one third higher than steel (modulus of elasticity 303 GPa, shear modulus 135 GPa, compression modulus 110 GPa). However, at 16.45 J / (mol · K), the molar heat capacity is significantly smaller than that of most other metals. The vibration damping is very high. It reflects around 50% in visible light and near ultraviolet, and around 98% in the infrared range at a wavelength of 10.6 µm. The speed of sound in beryllium is 2.5 times higher than that of steel, the coefficient of thermal expansion at room temperature is around 11 · 10 −6 per K. Since it has only four electrons per atom, the interaction with X-rays is very low. It is therefore very transparent to X-rays and is used as an exit window in X-ray tubes. Alpha radiation can release neutrons from beryllium :
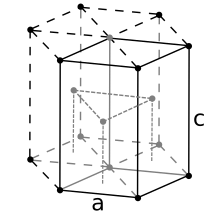
Beryllium usually crystallizes in a hexagonal close packing , called α-beryllium in the space group P 6 3 / mmc (No. 194) , in contrast to β-beryllium with a body-centered cubic form that ranges between 1250 ° C and its melting point of 1287 ° C is stable.
Beryllium has an extraordinarily low Poisson's number of µ = 0.032, so it shows very little transverse contraction in the tensile test, while other element metals have values from µ = 0.21 ( chromium ) to 0.44 or 0.45 ( gold , lead ; thallium ) exhibit. This means that a beryllium tensile specimen hardly constricts in the uniaxial tensile test, i.e. that its cross-section remains almost constant.
Chemical properties
Beryllium is one of the rarer metals that occurs in salts both cationic (beryllium silicates) and anionic (beryllates).
Beryllium and its compounds are similar in many respects to aluminum and its compounds due to their pronounced oblique relationship in the periodic table . At room temperature, beryllium is stable in dry air and remains bright because a passivating oxide layer is formed. Only when it is heated in powder form does it burn to form beryllium oxide and beryllium nitride with a bright appearance of fire. The oxide layer also resists attack by cold oxidizing acids, e.g. B. concentrated nitric acid up to a concentration of 6 M. Impurities with halide and sulfate ions promote the solution. In dilute, non-oxidizing acids (e.g. hydrochloric acid , sulfuric acid and ammonium hydrogen difluoride ), it dissolves vigorously with the evolution of hydrogen according to its strongly negative normal potential (−1.847 V).
Alkalis attack beryllium with the formation of berylates.
In humid air, it becomes coated with a layer of hydroxide that forms when it comes into contact with water. At higher temperatures, the corrosion resistance in water depends on the impurities in the metal and the corrosion medium; there is also the risk of pitting corrosion . In its pure form, it is not attacked by water, even when it is red heat. In hot gases such as air, oxygen , nitrogen and carbon dioxide , noticeable corrosion only occurs above 600 ° C.
In contrast to the other elements of main group II, beryllium dissolves, especially when heated, in aqueous alkali lye to form beryllates. When heated, it reacts with the halogens to form halides BeX 2 .
Few elements form substantial solid solutions in beryllium, namely copper , nickel, cobalt and, to a lesser extent, iron . Silver has limited solid solubility in beryllium. Most solid solution alloys are much harder than the cleaned metal; H. Metal from which micro-alloy-like contaminants have been removed. Beryllium forms many intermetallic compounds (for example with titanium ) that often play an important role in both alloy development studies and in the manufacture of beryllium composites . Aluminum is an important alloy additive that does not form an intermetallic compound with beryllium. Aluminum also has no significant solids solubility in beryllium and beryllium in aluminum, so that beryllium-aluminum alloys appear as mixtures of the two essentially pure metals.
Isotopes
A total of 11 isotopes between 5 Be and 16 Be of beryllium are known. Of these, only one, the isotope 9 Be, is stable. This makes beryllium one of 22 pure elements . The longest-lived unstable isotopes are 7 Be, which converts to 7 Li with a half-life of 53.22 days under electron capture and 10 Be, which decays to 10 B with a half-life of 1.51 million years under beta decay. Both isotopes are cosmogenic . All other isotopes only have short half-lives of seconds or milliseconds.
When used in nuclear reactors, gaseous products are produced by neutron capture and the subsequent nuclear reactions.
The proof of 10 Be is used, for example, in geology and climate research - in geology, for example, when dating the disclosure of rocks. This can be used, for example, to date the retreat of glaciers. The concentration of 10 Be shows a correlation with the cosmic rays reaching the earth. 10 Be arises from reactions of fast nucleons from cosmic radiation with nitrogen and oxygen in the air. The resulting spallation products adsorb on the aerosols in the upper atmosphere, which are finally transported with the rain to the earth's surface, where they mix with the stable 9 Be. The cosmic radiation and thus the rate of formation of 10 Be depend on the strength of the earth's magnetic field and the solar activity (high Be concentration with low solar activity). Since it preferentially precipitates on aerosol surfaces, high beryllium concentrations also correlate with high aerosol concentrations in the air. High concentrations occur in warm periods, low ones in cold periods. Since 10 Be is enclosed in ice cores together with the other gases in the atmosphere , the relationship between solar activity and global temperature history can be analyzed by analyzing these inclusions over many millennia.
The extremely short-lived isotope 8 Be (half-life about 10 −17 seconds) plays an important role in nucleosynthesis , the formation of the chemical elements in celestial bodies.
In 2008, the short-lived isotope 11 Be was found to have an interesting feature in terms of nuclear physics : its atomic nucleus consists of a relatively compact core and a single, loosely bound neutron that surrounds it as a halo .
use
The largest part (> 85%) of the beryllium produced worldwide is used for the production of various beryllium alloys (beryllium content less than 60%). About 10% is used for products made from pure beryllium and alloys with more than 60% beryllium content. The remaining beryllium is mainly used in beryllium oxide ceramics.
Based on value-added sales, around 22% of beryllium products were in industrial components, 21% in consumer electronics, 16% in automotive electronics, 9% in defense applications, 8% in telecommunications infrastructure, 7% in energy applications, 1% in medical applications and 16% in others Applications used.
beryllium
Despite the outstanding properties of beryllium, it is only used for a few applications due to its high price and health hazard.
Semi-finished products and raw parts made of beryllium metal are often manufactured as sintered products using powder metallurgy using HIP and CIP processes ( hot and cold isostatic pressing ). Cast parts made of beryllium are not used for technical purposes because of their anisotropic properties and other characteristics such as coarse grain.
Beryllium is used as a material for "windows" in X-ray tubes as well as X-ray and gamma radiation detectors because of the permeability for these rays, especially for the soft (low-energy) components. It is used for moderators and neutron reflectors in nuclear reactors and nuclear weapons , as neutron sources (together with an alpha emitter), in nuclear fusion systems such as JET ( Joint European Torus ) because of the high melting point and the small atomic number as a plasma delimiter ( English limiter ) and as mirror material especially for space telescopes because of the low weight and low coefficient of thermal expansion , for example in the James Webb space telescope .
Beryllium is also used for neutron multiplication in the blanket of future fusion reactors by means of the (n, 2n) nuclear reaction
intended. The combination of high neutron multiplication, low absorption and effective scattering offers very favorable properties for this.
In the European Synchrotron Radiation Facility , beryllium is used as a material for refractive lenses for X-rays (Compound Refractive Lenses, CRL) up to an energy of 40 keV.
Beryllium is used in particle accelerators such as the Large Hadron Collider as a building material for vacuum-tight tubes in the detectors, since beryllium scatters particles flying through it less than other materials.
Due to its weight and its high heat capacity , beryllium was used in brake disks and other parts of the space shuttle (window fastenings and other parts that are thermally and mechanically highly stressed). Rotors in gyro compasses , movable mirrors in optical systems, drive systems in magnetic tape devices are also made from the metal. Tweeters for high-end loudspeakers are made from beryllium metal (from 1974 to 1997 in the Yamaha Corporation ), as a dome membrane for ultra-high tones and now successfully for high-end dome tweeters in series production ( FOCAL TBe line,) coaxial speakers with dome Tweeters and cone mid-range speakers made of beryllium (TAD Labs) are used.
Beryllium is also used for motorsport (e.g. Porsche 906 ). Mercedes - Ilmor , supplier to the McLaren Formula 1 team, used this material in engine construction. The material was banned after a protest by Ferrari in 2001. The reason given was that the material is harmful to health during processing.
Since beryllium in stars is largely converted into other elements by the energy production processes, it is suitable as a marker for determining the age of stars.
Beryllium alloys
Beryllium is used as a construction material in alloys with aluminum for stressed and very light products in aircraft and space technology. Beralcast (formerly Lockalloy ) and AlBeMet-AM162 (62% Be, 38% Al) are brand names for fine powders from which the components are manufactured by hot isostatic pressing . 70 to 80% of the beryllium produced worldwide is used as an alloy component in beryllium copper (CuBe, CuCoBe). Among other things, spark-free, non-magnetic tools that can be used in potentially explosive areas are manufactured from this. Contact and spring materials made of beryllium copper are characterized by great hardness, elasticity , tensile strength , fatigue resistance , corrosion resistance , non-magnetizability and good electrical and thermal conductivity . Beryllium copper can therefore be used for contact springs or other current-transmitting springs, e.g. B. in moving coil measuring mechanisms or on carbon brushes , as well as for non-magnetizable tools for use in strong magnetic fields, for example for work on MRI machines. Beryllium copper can be found in precision sockets for ICs and as a material for the cans of can barometers due to its high elasticity. It is also used in relay contact springs , serves as contact spring material in hollow banana plugs , is used as a material for valve guides and valve seats in engine construction ( internal combustion engines ) and as CuBe and CuCoBe electrodes for spot welding and for plastic spray nozzles . CuBe can also be used as a material for dynodes of photoelectron multipliers (including beryllium oxide as a coating material).
Beryllium is also used as an alloy component with proportions of around 0.0001–0.1% by weight to improve the strength and elongation behavior of fine wires (“ bonding wires ”) made of gold , which are used in the semiconductor industry to contact components on a wiring carrier .
Some watch springs are made of iron - nickel - beryllium, NiBe.
Nickel-beryllium alloys are used for temperature -loaded connecting elements such as thermostat switches and nickel-beryllium tools because of their anti-sticking tendency for secondary boron-silicate glasses and varifocal optical lenses .
proof
In addition to atomic spectroscopic methods, beryllium can also be detected wet-chemically. Beryllium is determined as beryllium hydroxide (by precipitation with ammonia ) after interfering ions have been removed with quinolin-8-ol or masked by ethylenediaminetetraacetic acid . For photometric determination, the complexes are with pentane-2,4-dione , Morin, Thorin , Aluminon , chromeazurol and quinalizarin.
- With quinalizarin , a poorly soluble blue complex is formed in alkaline solution. To distinguish it from a similar looking magnesium compound, the beryllium complex is destroyed by bromine water in the presence of NaOH , while the Mg complex is stable for a time. In the presence of ammonia , the beryllium complex remains stable when bromine water is added, while the Mg complex is quickly destroyed.
- In an alkaline solution, Be (II) salts form a fluorescent colored lake with Morin . The analog aluminum compound does not fluoresce under these conditions. If acid is slightly acidified, the fluorescence of the Be compound disappears while the Al compound fluoresces.
Common analytical techniques include inductively coupled plasma atomic emission spectroscopy (ICP-AES), inductively coupled plasma mass spectroscopy (ICP-MS) and atomic absorption spectroscopy (AAS). In addition, a molecular fluorescence method for beryllium was developed that has a sensitivity comparable to ICP-MS. A large number of alternative techniques were also tried out. These include laser-induced breakthrough spectroscopy (LIBS), microwave -induced plasma spectroscopy (MIPS), aerosol transit time mass spectroscopy (TOFMS) and surface- enhanced Raman spectroscopy . In general, these techniques require significantly less sample preparation. However, due to problems with lack of precision at lower analyte values and the inadequate ability to process surface wipes, these methods have not caught on.
toxicology
Beryllium and beryllium compounds can cause allergic contact eczema. They can lead to a granulomatous skin reaction or a granulomatous disease with an immunological origin on the skin or lungs. In contrast to most other known allergic reactions in the airways, this is very likely due to a cell-mediated immunological reaction of the late type. When beryllium or its compounds are inhaled, the action of the macrophages produces primarily soluble beryllium oxide, which circulates ( hapten ), changes the structure of the body's own proteins and acts as an allergen if there is a corresponding genetic disposition . Current studies indicate that beryllium is not acutely toxic in the strict sense, but that the effects are the result of an excessive allergic reaction. The carcinogenic effect has also not been clearly proven.
Soluble beryllium compounds can lead to pronounced, poorly healing dermatitis due to the irritation . If beryllium salt solutions or undissolved particles get into the skin as a result of injuries or a damaged skin barrier, ulcers or necrosis can result. Up to the middle of the 20th century, more frequent reports of allergic contact dermatitis from beryllium salts and less often from beryllium oxide and beryllium were reported from companies producing beryllium or from companies processing beryllium. During this time, occupational cuts from fluorescent tubes, which z. B. containing zinc-manganese-beryllium-silicates, responsible for keloid-like, poorly healing or scarring granulomas due to beryllium salts or beryllium oxide that got into the skin.
The effects of beryllium accumulate in the human body and lead to serious illnesses after years of latency . Inhaled beryllium or inhaled beryllium salts are particularly dangerous - especially beryllium fluoride or beryllium oxide in high concentrations. This can lead to acute inflammation of the respiratory tract in the form of tracheobronchitis and pneumonitis , and in severe cases to pulmonary edema . Except in particularly severe cases, the symptoms with dyspnoea , cough and chest pain are mostly completely reversible. This form of disease was first observed in employees in the extraction and enrichment of beryllium or beryllium compounds from beryllium-containing ores. After a latency period of up to 5 years and more (in individual cases up to 30 years), a small proportion of those affected also developed a chronic lung disease ( berylliosis ). This usually occurs as a result of chronic exposure to low concentrations of beryllium. The disease can be detected by a lymphocyte transformation test.
Characteristic epithelial cell granulomas are formed in the lungs . Ingested beryllium is relatively harmless, as it is mostly excreted again.
safety instructions
In 2013, beryllium was included in the EU's ongoing action plan ( CoRAP ) in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. Beryllium uptake was caused by concerns about other exposure / risk-based concerns. The reassessment took place from 2013 and was carried out by Germany . A final report was then published in which it was determined that beryllium, due to its classification as carcinogenic category 1B and STOT RE1 (beryllium), meets the requirements for SVHC substances (Substances of Very High Concern) and should be classified accordingly.
Alloys containing beryllium - in solid form and as they are contained in the end products - do not present any particular health risks. However, in some manufacturing, processing and recycling processes, floating particles (dust, mist or smoke) are created, which, if inhaled, can lead to serious lung diseases. When processing beryllium, suction and encapsulation is therefore essential when removing chips. When electronic components containing beryllium oxide are destroyed, beryllium oxide can be released; they must therefore be labeled accordingly, which is often not the case, especially with older components.
links
In its compounds with electronegative binding partners, beryllium has almost exclusively the +2 oxidation state . The oxidation state +1 has it as an exception, for example, in the accessible at very high temperature by the reaction of beryllium with beryllium chloride and susceptible to decomposition at room temperature Berylliummonochlorid .
The beryllium cation Be 2+ is the most strongly polarizing ion in the alkaline earth group , so that the bonds with beryllium are extremely covalent . Because of this, beryllium does not form any BeX 2 compounds (X = electronegative residue) with predominantly ionic bond character. Like the covalent boron compounds , the covalent beryllium compounds also belong to the electron-unsaturated compounds which, in the case of monovalent groups X, lead to molecules BeX 2 in which the beryllium only has one electron quartet. Similar to boron, albeit less pronounced, beryllium can also eliminate this deficit of electrons through adduct formation, through p π p π bonds and through three-center bonds .
The most important coordination number of beryllium is four, whereby it is usually present as a tetrahedron as in beryllium chloride, but also square-planar as in beryllium phthalocyanine. In compounds BeX 2 with bulky substituents X or in the gaseous state, beryllium also occurs in its compounds with the coordination number three (trigonal planar), two (linear, as in gaseous beryllium chloride) and one (beryllium oxide in the gas phase). In exceptional cases, higher coordination numbers such as six or seven are also observed.
Oxygen compounds
In the total commercial volume, beryllium hydroxide is the most important beryllium compound. It precipitates out when bases are added to beryllium salt solutions . It arises as a product of the extraction processes for beryl and bertrandite ores and is used as an intermediate product for the production of metallic beryllium, beryllium oxide and beryllium-containing alloys as commercial products. Beryllium hydroxide is the most important compound for the production of high-purity beryllium oxide, which is used due to the ceramic properties of sintered beryllium oxide for the production or protection of materials that are used at high temperatures in corrosive environments - for example in lasers and electronics, space travel and nuclear technology.
Processes for the production of beryllium metal and for alloying with copper and / or nickel use beryllium hydroxide and beryllium oxide as the starting material. Beryllium oxide itself is used as a highly thermally conductive insulator for high-frequency power transistors , circulators and high-load resistors. Because of its toxicity, beryllium oxide is replaced with aluminum oxide , boron nitride or aluminum nitride whenever possible .
Halogen compounds
The colorless beryllium dihalides BeX 2 (X = F , Cl , Br , I ) can be prepared, for example, directly from the elements or by reacting HX with beryllium or X 2 with beryllium oxide in the presence of carbon . Beryllium fluoride is used in nuclear technology and as an intermediate in the production of pure beryllium.
Hydrogen compounds
The white, non-volatile, highly polymeric beryllium hydride BeH 2 (beryllan) can be produced by reacting dimethylberyllium with diborane . With trimethylamine and dimethylamine , beryllium dihydride forms amides such as the trimeric beryllium diamide . The colorless beryllium boronate, which flames in air and reacts explosively with water, can be obtained from beryllium chloride and lithium borohydride .
More connections
Beryllium forms a large number of other inorganic, organic and intermetallic compounds . They are mostly used for the production of beryllium or other beryllium compounds.
With nitrogen it forms the crystalline colorless and very hard beryllium nitride Be 3 N 2 at high temperatures . In addition, there is a higher nitrogen-rich nitride, the colorless beryllium diazide BeN 6 . When beryllium oxide and carbon react, the brick-red beryllium carbide Be 2 C is formed. With boron, it forms a series of beryllium borides Be n B m (Be 5 B, Be 4 B, Be 2 B, BeB 32 ). Structurally interesting is a sublimable, basic beryllium nitrate Be 4 O (NO 3 ) 6 that forms when anhydrous beryllium nitrate Be (NO 3 ) 2 is heated . Its structure corresponds to a Be 4 tetrahedron, the center of which is occupied by an oxygen atom and the six Be 2 edges of which are bridged by six (angled) nitrate ions. With acetic acid the beryllium compounds beryllium acetate Be (C 2 H 3 O 2 ) 2 or basic beryllium acetate Be 4 O (CH 3 COO) 6 are formed .
Like aluminum or magnesium, beryllium compounds form alkyl and aryl derivatives. These are capable of forming ate complexes , which in turn can have catalytic properties. Some compounds (such as beryllium bis-pentane-2,4-dionate or basic beryllium acetate) can be distilled without decomposition and serve as an intermediate product for the purification of beryllium. Beryllium diorganyls BeR 2 (R e.g. Me , Et , Pr iPr , Bu , tBu ...) exist as toxic, colorless, viscous liquids or solids. Like the former, they can be obtained by metathesis from beryllium chloride and RMgX or LiR and by transmetalation from beryllium and Hg R 2 .
→ Category: Beryllium compound
literature
- AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , pp. 1215-1224.
Web links
- What is the beryllium barrier? from the alpha-Centauri television series(approx. 15 minutes). First broadcast on Apr 13, 2005.
Individual evidence
- ↑ a b Harry H. Binder: Lexicon of the chemical elements. S. Hirzel Verlag, Stuttgart 1999, ISBN 3-7776-0736-3 .
- ↑ The values for the properties (info box) are taken from www.webelements.com (Beryllium) , unless otherwise stated .
- ^ IUPAC, Standard Atomic Weights Revised 2013 .
- ↑ Manjeera Mantina, Adam C. Chamberlin, Rosendo Valero, Christopher J. Cramer, Donald G. Truhlar: Consistent van der Waals Radii for the Whole Main Group. In: J. Phys. Chem. A . 113, 2009, pp. 5806-5812, doi: 10.1021 / jp8111556 .
- ↑ a b c d Entry on beryllium in Kramida, A., Ralchenko, Yu., Reader, J. and NIST ASD Team (2019): NIST Atomic Spectra Database (ver. 5.7.1) . Ed .: NIST , Gaithersburg, MD. doi : 10.18434 / T4W30F ( https://physics.nist.gov/asd ). Retrieved June 11, 2020.
- ↑ a b c d Entry on beryllium at WebElements, https://www.webelements.com , accessed on June 11, 2020.
- ^ NN Greenwood, A. Earnshaw: Chemistry of the elements. 1st edition. VCH, Weinheim 1988, ISBN 3-527-26169-9 , p. 136.
- ↑ Robert C. Weast (Ed.): CRC Handbook of Chemistry and Physics. CRC (Chemical Rubber Publishing Company), Boca Raton 1990, ISBN 0-8493-0470-9 , pp. E-129 to E-145. Values there are based on g / mol and given in cgs units. The value specified here is the SI value calculated from it, without a unit of measure.
- ↑ a b Yiming Zhang, Julian RG Evans, Shoufeng Yang: Corrected Values for Boiling Points and Enthalpies of Vaporization of Elements in Handbooks. In: Journal of Chemical & Engineering Data . 56, 2011, pp. 328-337, doi: 10.1021 / je1011086 .
- ↑ Ludwig Bergmann, Clemens Schaefer, Rainer Kassing: Textbook of Experimental Physics. Volume 6: Solids. 2nd Edition. Walter de Gruyter, 2005, ISBN 3-11-017485-5 , p. 361.
- ↑ a b Entry on beryllium in the GESTIS substance database of the IFA , accessed on April 30, 2017(JavaScript required) .
- ↑ Entry on beryllium in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ VS Kushneva: Spravochnik po Toksikologii i Gigienicheskim Normativam. IzdAT, Moscow 1999, ISBN 5-86656-092-5 , p. 23.
- ↑ a b Entry on beryllium in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ^ Laboratory Investigation. Vol. 15, 1966, p. 176.
- ^ Wilhelm Pape , Max Sengebusch (arrangement): Concise dictionary of the Greek language. 3rd edition, 6th impression, Vieweg & Sohn, Braunschweig 1914. 1914, accessed on July 23, 2020 .
- ^ Karl Ernst Georges : Comprehensive Latin-German concise dictionary . 8th, improved and increased edition. Hahnsche Buchhandlung, Hanover 1918 ( zeno.org [accessed July 23, 2020]).
- ↑ Uwe Ocken: The discovery of the chemical elements and the etymology of their names. From antiquity to alchemy to the atomic age . BoD - Books on Demand, 2018, ISBN 978-3-7460-5759-0 , pp. 129 ( limited preview in Google Book search).
- ↑ a b c d e f g h i j k l m n o p q r s t A. F. Holleman, N. Wiberg: Textbook of Inorganic Chemistry. De Gruyter, 2008, ISBN 978-3-11-020684-5 , p. 1216 (accessed from De Gruyter Online).
- ↑ See Annales de Chimie (1798), pp. 264 and 265 ( limited preview in Google book search).
- ^ Paddy Gannon: Revise AS Chemistry for AQA . Heinemann, 2005, ISBN 978-0-435-58318-7 , pp. 41 ( limited preview in Google Book search).
- ↑ Michael Clugston, Rosalind Flemming: Advanced Chemistry . OUP Oxford, 2000, ISBN 978-0-19-914633-8 , pp. 294 ( limited preview in Google Book search).
- ↑ a b c d e Beryllium and its compounds [MAK Value Documentation in German language, 2002.] In: The MAK Collection for Occupational Health and Safety. doi: 10.1002 / 3527600418.mb744041verd0034 .
- ^ Friedrich Kluge: Etymological dictionary of the German language . Walter de Gruyter GmbH & Co KG, 2012, ISBN 978-3-11-022365-1 , p. 152 ( limited preview in Google Book search).
- ↑ Jeffrey A. Hurlbut: The history, uses, occurrences, analytical chemistry, and biochemistry of beryllium - a review. December 16, 1974, DOW CHEMICAL USA, RFP-2152.
- ↑ a b c d e f g h i j k l m n o p q r Kenneth A. Walsh: Beryllium Chemistry and Processing . ASM International, 2009, ISBN 978-0-87170-721-5 , pp. 27 ( limited preview in Google Book search).
- ^ Disquisitio chemica de terra gemmarum, in Commentationes chemicae. Upsaliae 1777, p. 137.
- ↑ Determination of the constituents of some precious stones, Berlin 1779, p. 45.
- ^ Contributions to the chemical knowledge of the mineral bodies. Posen-Berlin, 1802, Vol. 3, pp. 215, 221.
- ↑ J. Mines 8, 1798, 533, Ann. Chim. 26, 1798, p. 155.
- ↑ See editorial footnote in the Annales de Chimie (1798) on p. 169, online .
- ↑ a b c d e f Martin Hosenfeld u. a .: 26. Gmelin's Handbook of Inorganic Chemistry. Beryllium. 8th edition. Verlag Chemie, Berlin 1930.
- ↑ Otto Linné Erdmann: Journal for practical chemistry . JA Barth, 1837, p. 249 ( limited preview in Google Book search).
- ↑ Marco Fontani, Mariagrazia Costa, Mary Virginia Orna: The Lost Elements The Periodic Table's Shadow Side . Oxford University Press, 2015, ISBN 978-0-19-938334-4 , pp. 78 ( limited preview in Google Book search).
- ^ Lothar Dunsch: Jöns Jacob Berzelius . Springer-Verlag, 2013, ISBN 978-3-322-94554-9 , pp. 73 ( limited preview in Google Book search).
- ^ Charles Lathrop Parsons: The Chemistry and Literature of Beryllium . BiblioBazaar, 2008, ISBN 978-0-559-26416-0 .
- ^ Johann Boillat: From Raw Material to Strategic Alloys. The Case of the International Beryllium Industry (1919-1939) . August 27, 2016, doi : 10.13140 / rg.2.2.35545.11363 ( researchgate.net [accessed January 3, 2018]).
- ↑ Bruce Cameron Reed: Atomic Bomb: The Story of the Manhattan Project How nuclear physics became a global geopolitical game-changer . Morgan & Claypool Publishers, 2015, ISBN 978-1-62705-991-6 , pp. 23 ( limited preview in Google Book search).
- ↑ a b c N. Krishna Rao, T. Sreenivas: Beryllium — Geochemistry, Mineralogy and Beneficiation. In: Mineral Processing and Extractive Metallurgy Review . 13, 1994, p. 19, doi: 10.1080 / 08827509408914098 .
- ^ A b c E. S. Grew, RM Hazen: Beryllium mineral evolution. In: American Mineralogist . 99, 2014, p. 999, doi: 10.2138 / am.2014.4675 .
- ↑ a b Rheinisch-Westfälisches Institut für Wirtschaftsforschung (RWI Essen), Fraunhofer Institute for Systems and Innovation Research (ISI), Federal Institute for Geosciences and Raw Materials (BGR): Trends in the supply and demand situation for mineral raw materials. 2006.
- ^ The Mineralogy of Beryllium. In: mindat.org. Hudson Institute of Mineralogy, accessed July 23, 2019 .
- ^ IMA: Mineral List with Database of Mineral Properties. Retrieved July 23, 2019.
- ↑ a b c usgs.gov: Beryllium Statistics and Information. Retrieved July 22, 2019.
- ^ KJ Schulz, John H. DeYoung, Robert R. Seal, Dwight C. Bradley: Critical Mineral Resources of the United States Economic and Environmental Geology and Prospects for Future Supply . Government Printing Office, 2018, ISBN 978-1-4113-3991-0 , pp. E-16 ( limited preview in Google Book search).
- ^ A b Michael J. Brisson, Amy A. Ekechukwu: Beryllium Environmental Analysis and Monitoring . Royal Society of Chemistry, 2009, ISBN 978-1-84755-903-6 , pp. 10 ( limited preview in Google Book search).
- ^ Smith C., Ingerman L., Amata R .: TOXICOLOGICAL PROFILE FOR BERYLLIUM . Ed .: US DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Agency for Toxic Substances and Disease Registry. Atlanta, GA, USA 2002 ( cdc.gov ).
- ↑ a b BeST: Facts and Figures. Retrieved August 3, 2019.
- ↑ Data sheet Beryllium wire, 0.25mm (0.01in) dia, annealed, 99.7% (metals basis) from AlfaAesar, accessed on August 3, 2019 ( PDF )(JavaScript required) .
- ^ Dennis R. Floyd, John N. Lowe: Beryllium Science and Technology . Springer, 2014, ISBN 978-1-4757-0668-0 , pp. 108 ( limited preview in Google Book search).
- ↑ Periodensystem.de: data on beryllium. Retrieved September 22, 2010.
- ↑ The anomalous molar heat capacity of beryllium is about 11 J / (K · mol) significantly lower than that of iron with 24.7 J / (K · mol). David Halliday, Robert Resnick: Physics. Part 2, Walter de Gruyter, Berlin / New York 1994, ISBN 3-11-013897-2 , p. 1455.
- ↑ Mark Winter: Poisson's ratio . In: Webelements.com.
- ↑ G. Audi, FG Kondev, Meng Wang, WJ Huang, S. Naimi: The NUBASE2016 evaluation of nuclear properties. In: Chinese Physics C . 41, 2017, p. 030001, doi: 10.1088 / 1674-1137 / 41/3/030001 ( full text ).
- ^ JM Kaste, SA Norton, CT Hess: Environmental Chemistry of Beryllium-7. In: Reviews in Mineralogy and Geochemistry . 50, 2002, p. 271, doi: 10.2138 / rmg.2002.50.6 .
- ↑ K. Horiuchi, EL Goldberg, K. Kobayashi, T. Oda, T. Nakamura, T. Kawai: Climate-induced variations of cosmogenic beryllium-10 in the sediments of Lake Baikal of the last 150ky from AMS, SRXRF and NAA data . In: Nuclear Instruments and Methods in Physics Research , Section A: Accelerators, Spectrometers, Detectors and Associated Equipment. 470, 2001, p. 396, doi: 10.1016 / S0168-9002 (01) 01085-3 .
- ^ RC Finkel, M. Suter : AMS in the earth sciences: technique and applications. In: Advances in Analytical Geochemistry . Volume 1, 1993, ISBN 1-55938-332-1 , pp. 1-114.
- ↑ a b Wilhelm T. Hering: Applied nuclear physics. Introduction and overview . Springer-Verlag, 1999, ISBN 978-3-519-03244-1 , pp. 62 ( limited preview in Google Book search).
- ^ Fritz Gassmann : What's wrong with the greenhouse earth . vdf, 1994, ISBN 3-7281-1935-0 , pp. 63 ( limited preview in Google Book search).
- ^ Richard Pott: General Geobotany: Biogeosystems and Biodiversity . Springer, 2005, ISBN 3-540-23058-0 , pp. 126 ( limited preview in Google Book search).
- ↑ JB Pedro, AM Smith, KJ Simon, TD van Ommen, MAJ Curran: High-resolution records of the beryllium-10 solar activity proxy in ice from Law Dome, East Antarctica: measurement, reproducibility and principal trends . In: Climate of the Past . tape 7 , July 2011, p. 707–721 , doi : 10.5194 / cp-7-707-2011 (English, clim-past.net [PDF; 1.7 MB ; accessed on July 16, 2013]).
- ↑ Elizabeth Vangioni, Michel Cassé: Cosmic origin of the chemical elements rarety in nuclear astrophysics. In: Frontiers in Life Science . 10, 2017, p. 84, doi: 10.1080 / 21553769.2017.1411838 .
- ↑ Atomic nucleus with halo: Scientists measure one-neutron halo with lasers for the first time. On: IDW online. February 16, 2009.
- ↑ a b c d e f g Ralph Puchta: A brighter beryllium. In: Nature Chemistry . 3, 2011, p. 416, doi: 10.1038 / nchem.1033 .
- ^ Beryllium related details from NASA. NASA, archived from the original on May 29, 2008 ; accessed on March 4, 2016 .
- ^ MW Werner, TL Roellig, FJ Low, GH Rieke, M. Rieke, WF Hoffmann, E. Young, JR Houck, B. Brandl: The Spitzer Space Telescope Mission . In: Astrophysical Journal Supplement . tape 154 , 2004, pp. 1 , doi : 10.1086 / 422992 , arxiv : astro-ph / 0406223 , bibcode : 2004ApJS..154 .... 1W .
- ^ The James Webb Space Telescope: The Primary Mirror.
- ↑ Pavel Vladimirov, Dmitry Bachurin a. a .: Current Status of Beryllium Materials for Fusion Blanket Applications. In: Fusion Science and Technology . 66, 2017, p. 28, doi: 10.13182 / FST13-776 .
- ↑ ESRF: Focusing compound refractive Beryllium lenses. Retrieved July 28, 2019.
- ↑ RXOPTICS: Lenses. Retrieved July 28, 2019.
- ↑ R. Veness, G. Simmons, C. Dorn: Development of beryllium Vacuum Chamber Technology for the LHC. (PDF; 444 kB). Proceedings of IPAC2011, San Sebastián, Spain 2011.
- ^ Lawrence Korb: Memories of the Apollo and Space Shuttle Programs . Page Publishing Inc, 2017, ISBN 1-68139-825-7 ( limited preview in Google Book Search).
- ↑ Yamaha Germany: NS-5000. Retrieved August 18, 2019.
- ↑ Focal.com: Beryllium tweeter. Retrieved August 18, 2019.
- ↑ TAD® PIONEER Professional Studio Loudspeaker Components: TAD® PIONEER Professional Studio Loudspeaker Components. Retrieved August 18, 2019.
- ^ MotorSportMagazine.com: The secrets to Ferrari's consistency. Retrieved July 27, 2019.
- ↑ Ifa.hawaii.edu: The Case of the Missing Beryllium. Retrieved July 26, 2019.
- ↑ a b entry on beryllium. In: Römpp Online . Georg Thieme Verlag, accessed on August 3, 2019.
- ↑ Nnamdi Anyadike: Copper A material for the New Millennium . Elsevier, 2002, ISBN 978-1-85573-870-6 , pp. 121 ( limited preview in Google Book search).
- ^ A. Davidson: Handbook of Precision Engineering Mechanical Design Applications . Macmillan International Higher Education, 2016, ISBN 978-1-349-01023-3 , pp. 121 ( limited preview in Google Book search).
- ↑ Tim Gilles: Automotive Engines: Diagnosis, Repair, and Rebuilding . Cengage Learning, 2018, ISBN 978-1-337-67022-7 , pp. 241 ( limited preview in Google Book search).
- ↑ Shu Tao, Hong Chan, Harry van der Graaf: Secondary Electron Emission Materials for Transmission Dynodes in Novel Photomultipliers: A Review. In: Materials. 9, 2016, p. 1017, doi: 10.3390 / ma9121017 .
- ^ JH Richardson: Systematic Materials Analysis . Elsevier, 2012, ISBN 0-323-14756-9 , pp. 115 ( limited preview in Google Book search).
- ↑ Patent application DE19733954A1 : Fine wire made of a gold alloy, method for its production and its use. Registered on August 6, 1997 , published on January 14, 1999 , applicant: Heraeus , inventor: Günther Herklotz, Jürgen Reuel, Lutz Schräpler, Christoph Simons.
- ^ A b Joseph R. Davis: Nickel, Cobalt, and Their Alloys . ASM International, 2000, ISBN 978-0-87170-685-0 , pp. 102 ( limited preview in Google Book search).
- ↑ Gerhart Jander, Ewald Blasius: Textbook of analytical and preparative inorganic chemistry. 14th edition, Hirzel, Stuttgart 1995, pp. 428-429.
- ↑ Gerhart Jander, Ewald Blasius: Textbook of analytical and preparative inorganic chemistry. 14th edition, Hirzel, Stuttgart 1995, pp. 632-633.
- ↑ a b Werner Böcker, Helmut Denk, Philipp U. Heitz, Holger Moch, Gerald Höfler, Hans Kreipe: Textbook Pathology . Elsevier Health Sciences, 2019, ISBN 3-437-17143-7 , pp. 69 ( limited preview in Google Book search).
- ↑ Dominik Naglav, Magnus R. Buchner, Georg Bendt, Florian Kraus, Stephan Schulz: Off the Beaten Track - A Hitchhiker's Guide to Beryllium Chemistry. In: Angewandte Chemie International Edition . 55, 2016, p. 10562, doi: 10.1002 / anie.201601809 .
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): Beryllium , accessed on May 20, 2019.
- ^ European Chemicals Agency (ECHA): Substance Evaluation Report and Conclusion Document .
- ^ Beryllium Science & Technology Association: Beryllium-Containing Materials. Forging, exposure control, guideline.
- ↑ a b c NCBI Bookshelf: Arsenic, Metals, Fibers and Dusts. Beryllium and Beryllium Compounds. Retrieved July 25, 2019.
- ↑ Entry on beryllium compounds. In: Römpp Online . Georg Thieme Verlag, accessed on August 3, 2019.






![{\ displaystyle {\ ce {Al2Be3 [Si6O18] + 6 H2SO4 -> 3 BeSO4 + Al2Si6O12 (SO4) 3 + 6 H2O}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/33e8fe20d31b54d4526f931c5a6454f1b357d510)

![{\ displaystyle {\ ce {Al2Be3 [Si6O18] + 6 Na2SiF6 -> 3 Na2BeF4 + 2 Na3AlF6 + 9 SiO2 + 3 SiF4}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/f93130c018cdc4c1daf0013d9a4a245ccca49b0f)


















