sulfur
| properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name , symbol , atomic number | Sulfur, S, 16 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Element category | Non-metals | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group , period , block | 16 , 3 , p | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | yellow | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number | 7704-34-9 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EC number | 231-722-6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ECHA InfoCard | 100,028,839 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC code | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mass fraction of the earth's envelope | 0.048% | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic mass | 32.06 (32.059-32.076) u | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius (calculated) | 100 (88) pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 102.5 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 180 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [ Ne ] 3 s 2 3 p 4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1. Ionization energy | 10.36001 (12) eV ≈ 999.59 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2. Ionization energy | 23.33788 (25) eV ≈ 2 251.76 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3. Ionization energy | 34.86 (4) eV ≈ 3 363.48 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4. Ionization energy | 47.222 (12) eV ≈ 4 556.23 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5. Ionization energy | 72.5945 (4) eV ≈ 7 004.3 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6. Ionization energy | 88.0529 (4) eV ≈ 8 495.81 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physically | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical state | firmly | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | orthorhombic | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| density | 2.07 g / cm³ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| magnetism | diamagnetic ( Χ m = −1.3 10 −5 ) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 388.36 K (115.21 ° C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| boiling point | 718.2 K (445 ° C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar volume | 15.53 · 10 −6 m 3 · mol −1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of evaporation | 45 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 1.713 kJ mol −1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Specific heat capacity | 736 J kg −1 K −1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 0.205 W m −1 K −1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemically | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | ± 2, 4, 6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal potential | −0.48 V (S + 2 e - → S 2− ) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 2.58 ( Pauling scale ) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| For other isotopes see list of isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NMR properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| safety instructions | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Schwefel (via Middle High German swëbel from Old High German swëbal ; Latin sulpur and Greek sulphur or Sulfur , like swëbal presumably from an Indo-European root suel with the meaning 'burn slowly', from which Germanic also German 'smolder' arose; the name for compounds containing sulfur The syllable "-thio-" used comes from the Greek word θεῖον theĩon "sulfur") is a chemical element with the element symbol S and the atomic number 16. It is one of the chalcogens , the sixth main group of the periodic table . In terms of the abundance of elements in the lithosphere , it is in 16th place. Sulfur is a yellow, non-metallic solid that forms a variety of allotropic modifications. In nature it is both dignified and in form of its inorganic compounds in this mainly as a sulfide or sulfate .
Certain amino acids and coenzymes contain sulfur; in organisms it plays a role in the anaerobic energy production of microorganisms. The chemical industry uses most of the elemental sulfur to produce sulfuric acid , one of the technically most important and most widely produced basic chemicals . As a component of acid rain , sulfur oxides are of considerable environmental relevance.
history
Sulfur has been an element that humans have used for a long time. The Chinese and Egyptians used around 5000 BC Chr. Sulfur for bleaching textiles, as a medicine and for disinfection. The Ebers papyrus describes the use of sulfur to treat bacterial inflammation of the eye ( trachoma ).
A naturally occurring modification of sulfur called Shiliuhuang has been around in China since the sixth century BC. Known. The Chinese extracted sulfur from pyrite in the third century BC .
Pre-classical Greece used sulfur as a medicine and the sulfur dioxide produced by burning sulfur both as a disinfectant to prevent infectious diseases such as the plague and to add sulfur to wine . Already around 800 BC Homer mentioned this in the Odyssey . Ancient warfare used sulfur as an incendiary weapon or fire accelerator . Around 79 AD, Pliny the Elder , in his work Naturalis historia, mentioned the island of Milos as a depository of the element and its use as a disinfectant, medicine and bleach. He mentions sulfur woods as other uses .
A treatise from the Song Dynasty around 1044 describes various forms of Chinese black powder , a mixture of potassium nitrate , charcoal and sulfur. Roger Bacon described the preparation of a similar mixture in 1242. For a long time, black powder remained the only explosive and explosive substance . The role of the German monk Berthold Schwarz , to whom the rediscovery of black powder is usually attributed, has not been clearly documented historically.
In the Middle Ages, a distinction was made between ordinary, not yet heated sulfur (called "living sulfur", Middle Latin sulfur vivum ) and sulfur sublimatum .
The environmental impact of sulfur oxides from the burning of coal on air quality in London was described by John Evelyn in a letter to Charles II in 1661 and in his work Fumifugium (The Inconveniencie of the Aer and Smoak of London Dissipated) , the first book on the Air pollution in London.
As one of the first chemical-technical processes, John Roebuck developed the lead chamber process for the production of sulfuric acid from 1746 . In November 1777, Antoine Laurent de Lavoisier first suspected that sulfur was an element. His experiments and observations on the combustion behavior of sulfur ultimately led to the case of the phlogiston theory . Nevertheless, in 1809 Humphry Davy came to the result experimentally that sulfur contained oxygen and hydrogen. The ultimate proof of the elemental character was achieved in 1810 by Joseph Louis Gay-Lussac and Louis Jacques Thénard when reviewing Davy's experiments.
The element symbol S has been used since 1814, following a proposal by Jöns Jakob Berzelius , who included it in his atomic weight table under the name Sulfur. The Danish chemist William Christopher Zeise discovered about 1822, the xanthates and introduced in 1834 with ethanethiol the first mercaptan ago.
The contact process was developed and patented in 1831 by the vinegar producer Peregrine Phillips . In British Patent No. 6096 he describes the spontaneous oxidation of sulfur dioxide to sulfur trioxide in air in the presence of a platinum catalyst . Subsequent absorption of the trioxide in water resulted in sulfuric acid. Vanadium pentoxide subsequently replaced platinum as a catalyst. Another milestone in the development of chemical-technical processes was achieved by Charles Goodyear in 1839 with the discovery of the vulcanization of rubber with elemental sulfur; it forms the basis of the rubber industry. The process laid the foundation for the establishment of a tire empire by Frank and Charles Seiberling, who chose the name Goodyear in the company name in his honor.
Between 1891 and 1894, the German-born chemist Hermann Frasch developed the Frasch process , with which the underground sulfur deposits discovered in Louisiana in 1865 could be dismantled.
In 1912, Ernst Beckmann demonstrated cryoscopically that rhombic sulfur consists of S 8 rings. This proof was achieved by X-ray structure analysis in 1935 BE Warren and JT Burwell. From the 1970s onwards, Max Schmidt's work group produced new allotropic modifications of sulfur.
Occurrence
Terrestrial occurrence
Sulfur occurs in many spheres of the earth . The exchange between these spheres is described by the sulfur cycle , the system of conversions of sulfur and sulfur-containing compounds in the lithosphere , hydrosphere , earth's atmosphere and biosphere, as well as the exchange between these earth spheres. Sulfur occurs in the −2 oxidation state, for example in metal sulfides and hydrogen sulfide, and −1, for example in amino acids . The oxidation level 0 (elemental sulfur) occurs in sediments that come from the bacterial reduction of sulfates, such as in Louisiana, or in sulfur deposits of volcanic origin. In the +4 oxidation state it occurs as sulfur dioxide in the atmosphere and in the +6 oxidation state as sulphate in the hydro- and lithosphere.
In terms of the abundance of elements, sulfur has a mass fraction of
- 0.46% on the whole earth in 8th place,
- 0.048% in the earth's envelope in 15th position,
- 0.035% in the continental crust in 16th place.
Lithosphere
Elemental sulfur occurs naturally in huge deposits, for example in Sicily , Poland , Iraq , Iran , Louisiana , Texas and Mexico . Until 2011, native sulfur was found at around 1,500 sites worldwide. In addition to the deposits already mentioned, sulfur was found in several regions of Australia , North and South America , Asia and Europe . Sulfur was found in mineral samples from the ocean floor of the Gulf of Mexico , the Mid-Atlantic Ridge and the East Pacific Ridge .
Sulfur occurs native , ie in elemental form in nature. Pure sulfur is relatively rare overall, but volcanic eruptions release it in large quantities. It can be found in volcanic vents or on other post-volcanic phenomena as a resublimation product in powder form as a so-called sulfur bloom . However, sulfur produced synthetically by refining is also referred to as sulfur flower or sulfur blossom.
Elemental sulfur is recognized as an independent mineral and is used by the International Mineralogical Association (IMA) according to the systematics of minerals according to Strunz (9th edition) under the system no. "1.CC.05" (elements - semi-metals (metalloids) and non-metals - [group] sulfur-selenium-iodine) ( 8th edition : I / B.03-10 ). The systematics of minerals according to Dana , which is also common in English-speaking countries , leads the element mineral under the system no. "03/01/05/01".
The naturally occurring modifications β-sulfur and rosickýite (γ-sulfur) are also recognized as minerals.
Sulfur crystallizes monoclinically (β-sulfur) above around 95 ° C. Under standard conditions, this form quickly transforms into the then thermodynamically stable α-sulfur. The latter crystallizes in the orthorhombic crystal system in the space group Fddd (No. 70) with the lattice parameters a = 1044 pm ; b = 1284 pm and c = 2437 pm and 128 formula units per unit cell .
The density of sulfur is about 2.0 to 2.1 g / cm³ and its Mohs hardness is about 1.5 to 2.5. It usually shows light yellow to dark yellow crystal prisms or pyramid shapes that are formed on rock surfaces from sulfur-rich gases through incomplete oxidation of hydrogen sulfide (H 2 S) or reduction of sulfur dioxide (SO 2 ). Sulfur leaves a white line on a whiteboard .
Sulfur usually occurs in a coarse form, that is, without crystals that can be seen with the naked eye, especially in sediments or sedimentary rocks . It is often found in evaporites (salt rocks), where it is mostly formed by the reduction of sulfates .
Larger crystals are transparent to translucent, have a resinous to greasy sheen on their surfaces and have the following optical properties:
- Main indices of refraction : n α = 1.9579; n β = 2.0377 and n γ = 2.2452 (biaxially positive)
- Angle, dispersion of the optical axes: 2v z ≈ 68 ° 58 '
Powdery or massive aggregates , on the other hand, are opaque matt.
Dallol volcanic landscape with brown iron oxide and yellow sulfur in the Afar region of Ethiopia
Steaming sulfur fumaroles on White Island in New Zealand
Needle-like sulfur crystals on a fumarole near the Krafla volcano in Iceland
Light yellow sulfur on calcite
Depending on where it was found, sulfur can occur in paragenesis with various other minerals such as anhydrite , aragonite , calcite , celestine , gypsum and halite .
Much more often than in its native form, sulfur occurs naturally in bound form in various minerals, above all in sulfides and in oxides, halides and others. A total of almost 1,000 sulfur minerals were known in 2010. Is widely used sulfur in sulfide minerals such as pyrite and marcasite (FeS 2 ), chalcopyrite (CuFeS 2 ), galena (PbS) and sphalerite (ZnS). Heavy metals are often found in nature as poorly soluble sulphides . Sulfur occurs naturally in the form of sulphates, such as sulphate ions in the oceans (about 0.9 g / l), gypsum (CaSO 4 · 2 H 2 O), barite (BaSO 4 ) and other sulphates that are difficult to dissolve in water.
Minerals with the highest sulfur content are the sulphides patrónite (approx. 71.6%), villamanínite (approx. 55.9%), hauerite (approx. 53.9%), pyrite and marcasite (each approx. 53.5%).
Examples of sulfur halides are connellite and kleinite , for sulfur oxides hannebachite and kuzelite , for sulfur carbonates leadhillite and tychite , for sulfur sulfates cyanotrichite and swordmannite , for sulfur phosphates arsentsumebite and chalcophyllite and for sulfur silicates Haüyn and Nosean .
Fossil fuels such as petroleum , natural gas and coal are an important source for the extraction of sulfur . Natural gas in particular contains a relatively large amount of hydrogen sulfide (H 2 S). The sulfur content in lignite is up to 10%.
Hydrosphere
In the hydrosphere, sulfur usually occurs in the form of the sulfate ion; With a concentration of 7.68% of the total salt content, it is the third most common ion in sea water after the chloride and sodium ions. Marine microorganisms use sulfate to break down the methane present on the sea floor. The sulphate is reduced to hydrogen sulphide, which is oxidized again by other microorganisms in the higher ocean layers.
Sulphates from natural sources such as gypsum deposits occur in fresh water and contribute significantly to water hardness. According to the German Drinking Water Ordinance, a sulphate limit value of 240 mg / l applies to drinking water . Sulphate concentrations above 100 mg / l are considered to be corrosive and attack both steel and concrete structures.
the atmosphere
In the upper regions of the atmosphere, caused for example by volcanic eruptions, sulfur-rich particles are found as aerosols with particle sizes of 0.1 to 1 micrometer. As the particles reflect sunlight in the stratosphere , they are said to have a cooling effect on the global climate.
Due to the combustion processes of fuels containing sulfur, sulfur occurs as sulfur dioxide in the troposphere . Around 35% of the total sulfur dioxide emissions of around 400 million tons of SO 2 per year come from anthropogenic sources . The majority of organic sulfides come from marine phytoplankton , which mainly release dimethyl sulfide and hydrogen sulfide and are considered the second largest overall source of emissions for sulfur-containing particles.
The subject of the scientific debate on the climate crisis is to what extent the artificial introduction of sulfur into the atmosphere is a suitable geoengineering measure to counteract the overheating of the earth's climate system and thus to still be able to achieve the two-degree target agreed in the Paris Agreement . The atmospheric scientists Ulrike Niemeier and Simone Tilmes once said: "In order to keep the global temperature rise in check, the annual sulfur input into the stratosphere would have to correspond to the eruption of Pinatubo on June 12, 1991." In fact, the eruption of the Pinatubo , which emitted large amounts of sulfur, important findings. It could be concluded that a sulfur input that is large enough to compensate for the overheating of the earth's surface caused by the doubling of atmospheric CO 2 would have major consequences for the ozone layer . Tilmes warned of the risks of geoengineering from the introduction of sulfur into the atmosphere: "Our results show that this approach of artificially reducing global warming would entail major risks."
biosphere
Sulfur occurs in various forms in the biosphere, often in a reduced form. In the course of the breakdown of biomass by enzymes and microorganisms, hydrogen sulfide is released from organic substances. Sulfur is mainly available to plants in the form of anionic sulfate from the soil, which is absorbed and distributed via the roots and is mostly unmetabolized in the vacuolar sap . The reduction of sulfate to sulfide requires 732 kJ mol −1 . Sulfur dioxide, on the other hand, is easily absorbed and assimilated through leaves. Sulphate is a macronutrient whose deficiency can lead to yield losses in agricultural production.
From chemautotrophic , aerobic , sulfide-oxidizing bacteria , hydrogen sulfide is oxidized with oxygen to elemental sulfur. Hydrogen sulfide is used by phototrophic ( anaerobic ) bacteria under anoxic conditions in anoxygenic photosynthesis as a reducing agent for the assimilation of carbon dioxide and is oxidized to elemental sulfur or sulfate.
Stellar occurrences

Diatomic sulfur was first detected in the tail of the comet IRAS-Araki-Alcock (C / 1983 H1). After detection in other comets, it is now assumed that occurrence in comet tails is ubiquitous. The origin of the S 2 molecule has not been clarified.
The clouds of Venus consist largely of sulfur dioxide and sulfuric acid droplets. The sulfuric acid is formed photochemically in the upper atmosphere of Venus through the ultraviolet radiation of the sun from carbon dioxide and sulfur dioxide. The short-wave radiation releases oxygen from the carbon dioxide, which reacts with sulfur dioxide to form sulfuric acid by absorbing water.
The Viking probes discovered sulfur on Mars . The content of sulfur-containing compounds, mainly present as magnesium and iron sulphate, in the Martian dust was up to three percent by weight. The formation of the sulfates probably required an aqueous environment and is therefore interpreted as an indication of the presence of a prehistoric hydrosphere on Mars.
Numerous lakes of molten sulfur have been found on Jupiter's moon Io . The broad color spectrum of the sulfur deposits gives the moon a colorful appearance. In the case of the lava flows , which extend over several hundred kilometers, it is assumed that they mainly consist of sulfur or sulfur compounds.
Research by NASA researchers suggests that two-thirds of the salts on the surface of Jupiter's moon Europa could consist of sulfuric acid. Others believe the presumed ocean beneath the ice crust may be rich in sulfuric acid.
Interstellar occurrences
So far, astronomers have detected 13 different sulfur compounds in interstellar space. These include carbon sulfide (CS), sulfur monoxide (SO), silicon sulfide ( SiS), carbonyl sulfide (COS), hydrogen sulfide (H 2 S), thioformaldehyde (H 2 CS) and sulfur dioxide (SO 2 ). Astronomers hope to detect volcanism on extrasolar planets by detecting sulfur dioxide.
Most of the compounds were found in interstellar molecular clouds , the size, density and temperature of which allow the formation of molecules and protect them from high-energy radiation. The scientists were able to prove the connections by means of radio telescopy in the millimeter wavelength range.
Extraction
Sulfur is obtained either as elemental sulfur, more than 90% of which is further processed into sulfuric acid, or in the form of its oxide by roasting sulfidic ores. Elemental sulfur is extracted and traded worldwide. The largest production sites are in the United States of America , Canada , the former Soviet Union and Western Asia. The People's Republic of China is the world's largest importer, followed by Morocco and the United States. Canada is the largest exporter, followed by Russia and Saudi Arabia .
Mining geological sulfur deposits

Sulfur can be obtained from geological deposits of elemental crude sulfur or sulfur-containing compounds in hydrocarbon sources such as crude oil, natural gas and coal, as well as from sulphidic ores of heavy metals. In the form of sulfates, sulfur, for example as gypsum, is available in practically unlimited quantities. The currently economically accessible sources are estimated at 5 × 10 12 tons of sulfur. Another 600 × 10 12 t of sulfur are believed to be in the form of sulphurous coal, oil shale and sands. In the United States in 2007, the amount of elemental sulfur extracted was 8.2 million tons.
Fumaroles occur on and near volcanoes , which together with their gases emit hydrogen sulphide and gaseous elemental sulfur, which condenses at the outlet point when it cools and forms crystals. In the Middle Ages, deposits of such fumaroles in Iceland , such as Námafjall , were an important source for the manufacture of gunpowder throughout Europe. In Ijen , a volcanic complex in the Indonesian East Java , there is a solfatar , which is considered to be the largest sulfur deposit in Indonesia. The sulfur is broken out of the eight meter thick sulfur banks and transported from the crater with bamboo baskets.
Underground sulfur deposits were prepared by the of Herman Frasch developed Frasch process mainly in the USA and in Poland exploited. For this purpose, pipes are driven into the sulfur deposit through layers above. The sulfur is liquefied by injected steam and superheated water and is carried to the surface by forced air. In 1995, the annual production using this process was 3.1 million tons. However, the economically viable deposits have become rare. In the US, production of the last deposit using this method was discontinued in 2001.
Sulfur recovery
Today, sulfur is produced in large quantities as a waste product from the separation of hydrogen sulfide from natural gases and the desulfurization of crude oil using the Claus process . Natural gas contains up to 35% hydrogen sulfide, crude oil contains about 0.5 to 1% sulfur in low-sulfur form, depending on the occurrence the content is up to 5% sulfur. The basic chemical process of sulfur recovery consists of two steps: In the first step, a third of the hydrogen sulfide is burned to form sulfur dioxide. The remaining two thirds of the hydrogen sulphide react with the sulfur dioxide (SO 2 ) by comproportioning to form sulfur.
Production as sulfur dioxide
The sulfidic ores of iron , copper , zinc , lead and other metals are roasted in air to form metal oxide and sulfur dioxide . The sulfur dioxide produced is oxidized to sulfur trioxide by catalytic oxidation and processed directly into sulfuric acid. The pyrite representation can be described in a simplified manner using the following equations:
When the pyrite is heated in the absence of air, elemental sulfur is obtained. The process was already known in the Middle Ages.
Storage and distribution
In the Claus process, sulfur is produced in liquid form and is usually stored and transported that way. This has a number of cost and quality advantages over dealing with solid sulfur, because solid sulfur often has to be liquefied before it can be used. When storing solid sulfur, unwanted sulfuric acid can be formed both by air humidity and by sulfur bacteria. Iron sulfide formed by corrosion has a finely divided pyrophoric effect and can cause fires or explosions.
Liquid sulfur is bottled at 135 to 140 ° C; the temperature during transport must not fall below 118 ° C or exceed 160 ° C. It is kept in a liquid state by heating with low-pressure steam of 3 to 4 bar and transported by ship, in tank cars or in specially equipped tankers . Previously, liquid sulfur must be freed from hydrogen sulfide as much as possible. Complete degassing is usually not possible. If liquid sulfur is handled openly, this leads to odor nuisance. Due to the high coefficient of thermal expansion, sulfur frozen in pipelines must be thawed carefully - specifically: slowly progressing in space - since defrosting too quickly can lead to the pipe bursting.
Physical Properties
The physical properties of sulfur are strongly dependent on temperature, as a number of allotropic modifications can be present at a given temperature. If sulfur is heated to over 119 ° C, a low-viscosity liquid of light yellow color is formed, in which mainly S 8 rings are present. If the temperature is maintained, a partial conversion of the S 8 rings into smaller rings leads to a lowering of the melting point , which has its minimum at 114.5 ° C. On further heating, the viscosity increases further and reaches its maximum at 187 ° C. The sulfur rings break open and form long-chain molecules, an example of ring-opening polymerization . Above this temperature the chains break down again into smaller fragments and the viscosity decreases again.
Sulfur is the element with the most inter- and intramolecular allotropic modifications . Intermolecular allotropes are solid-state phases of an element that differ in their crystal structure. About 30 different allotropes are known to date. The forms, which are thermodynamically stable under normal conditions, all consist of S 8 rings. There are also a number of intramolecular allotropes in the form of rings of different sizes and chains of different lengths.
Cyclooctasulfur
Naturally occurring solid sulfur consists of S 8 - molecules , in which the sulfur atoms are annularly arranged in a so-called crown shape. Cyclooctasulfur occurs in three intermolecular allotropes.
The most thermodynamically stable modification of sulfur at room temperature is orthorhombically crystallizing α-sulfur. It is odorless and tasteless and has the typical sulfur yellow color. α-sulfur crystallizes orthorhombically in the space group Fddd (No. 70) with the lattice parameters a = 1044 pm , b = 1284 pm and c = 2437 pm as well as 16 formula units S 8 per unit cell .
α-sulfur occurs gediegen as flowers of sulfur (yellow sulfur) in nature, has a density of 2.0 g / cm³ to 2.1 g / cm³, a hardness of 1.5 to 2.5 and a light to dark yellow Color and a white line color . It usually shows light yellow prismatic or pyramid-shaped crystals that are formed on rock surfaces from sulfur-rich gases due to incomplete oxidation of hydrogen sulfide or reduction of sulfur dioxide .
The transition point to β-sulfur is at 95.6 ° C. This sulfur modification is almost colorless and crystallizes monoclinically in the space group P 2 1 / a (No. 14, position 3) with the lattice parameters a = 1085 pm ; b = 1093 pm; c = 1095 pm and β = 96.2 ° as well as 6 formula units S 8 per unit cell. If β-sulfur is heated to 100 ° C and quickly cooled to room temperature, this modification is stable for several weeks.
The monoclinic crystallizing γ-sulfur ( rosickýite ) with the space group P 2 / c (No. 13) and the lattice parameters a = 844 pm is rarer ; b = 1302 pm; c = 936 pm and β = 125.0 ° as well as 4 formula units S 8 per unit cell. The mineral is found in Death Valley in the USA, where it is created and stabilized by the microbiological reduction of sulfate.
Cyclohexasulfur
Cyclohexasulfur S 6 , known as Engels sulfur, exists in a chair conformation with an S – S bond length of 2.057 ± 0.018 Å and an S – S – S bond angle of 102.2 ± 1.6 ° with high ring strain . The orange-colored, rhombohedral crystals can be made by various methods. Engel produced cyclohexasulfur as early as 1891 by acidifying a sodium thiosulfate solution with hydrochloric acid .
The density is 2.21 g / cm³, the melting point is around 100 ° C. Under normal conditions, cyclohexasulfur converts to cyclooctasulfur within a few days.
Cycloheptasulfur
Cycloheptasulfur can be produced by reacting cyclopentadienyl titanium pentasulfide with disulfur dichloride .
- Cp = η 5 -C 5 H 5
Depending on the manufacturing conditions, cycloheptasulphur exists in four different intermolecular allotropic modifications (α-, β-, γ-, δ-cycloheptasulphur). These are all temperature-sensitive and quickly transform into the thermodynamically stable form at temperatures above 20 ° C. However, the modifications can be kept for a longer period of time at a temperature of −78 ° C. The sulfur-sulfur bond lengths occurring in the ring are between 199.3 and 218.1 pm.
Larger sulfur rings
Larger sulfur rings (S n with n = 9-15, 18, 20) can be formed using the cyclopentadienyl titanium pentasulfide method or by reacting dichlorosulfanes S m Cl 2 with polysulfanes H 2 S p .
There are four intermolecular allotropes of cyclononasulfur S 9 , two of which, called α- and β-cyclononasulfur, are characterized.
With the exception of the cyclododecasulfur S 12 , the intramolecular bond lengths and angles in the sulfur modifications are different. Cyclododecasulfur is the most stable modification after cyclooctasulfur. There are two intermolecular modifications of cyclooctadecasulfur S 18 as ring conformational isomers .
Polymeric sulfur
Polymeric sulfur consists of long polymeric sulfur chains called catenapoly-sulfur. The nature of the end group is not known. The polymer is obtained by heating to temperatures above 120 ° C and then rapid cooling in cold water or liquid nitrogen. The maximum chain concentration is found at temperatures between 250 and 300 ° C. Existing sulfur rings can be extracted with carbon disulfide.
Liquid sulfur
The β-sulfur melts when heated to 119.6 ° C. The melt initially consists of cyclooctasulphur molecules, so-called λ-sulfur (sulfur bloom, S λ ). After a while, an equilibrium is established between the various intramolecular allotropes in the melt, with other rings (especially S 6 , S 7 , S 12 ) appearing as a function of temperature. There are also much larger rings such as S 50 and chain structures at higher temperatures.
As the temperature rises further, the concentration of the smaller rings initially increases and the viscosity decreases, so-called π-sulfur with S n (6 ≤ n ≤ 25, n ≠ 8). From a temperature of 159 ° C, the so-called λ transition begins, at which the rings break open due to thermal excitation and form long chains due to polymerization. The polymeric sulfur reaches its maximum viscosity at 187 ° C. A number of physical properties change at the λ transition, for example viscosity, optical absorption and thus also color. So-called ω-sulfur is present. If this is cooled down quickly, after extraction with carbon disulfide, it is in solid form as amorphous, plastic μ-sulfur, with S n (10 3 ≤ n ≤ 10 6 ).
Further heating to the boiling point of 444.6 ° C causes the chains to break down again into smaller fragments and the viscosity of the melt decreases.
Gaseous sulfur
Gaseous sulfur is dark red and initially consists of S 8 rings, which continue to break at higher temperatures. At a temperature of 330 ° C, the steam consists mainly of cycloheptasulfur S 7 . At temperatures above 550 ° C the rings disintegrate into smaller molecules such as S 2 - 4 . Above 700 ° C the steam mainly contains S 2 molecules and at 1800 ° C sulfur is present in the form of individual atoms .
The heating of the gaseous sulfur is associated with intense color changes. Initially, the sulfur vapor has the same yellow color as a cyclooctasulphur melt. With further heating, the color changes from yellow to orange and dark red to dark red-brown. This is the presence of linear S 2-4 due species.
Charged sulfur molecules
Neutral sulfur molecules can change the oxidation state through oxidation or reduction . Sulfur molecules having the +2 oxidation state, for example, the bright yellow S 4 2 + - cations , the red S 16 2+ cations and the S 8 2+ cations. These charged sulfur molecules are obtained by reacting with arsenic pentafluoride or antimony pentafluoride :
The ring structure of the sulfur molecules is retained, but the original conformation changes.
Cyclooctasulfur can be reduced by sulfide ions to an open-chain nonasulfide ion with the oxidation state −2. However, this charged sulfur molecule is not stable and easily splits into shorter chain-like polysulfides .
Such sulfur anions can be produced in anhydrous form by reduction with base metals. In this case too, the cyclic structure is transformed into a chain structure.
Chemical properties
Sulfur is a reactive element and reacts at elevated temperatures with many metals apart from platinum , iridium and gold to form sulfides. With mercury sulfur reacts already on trituration at room temperature to mercury sulfide . Sulfur reacts with semimetals and non-metals at elevated temperatures. Exceptions are tellurium , molecular nitrogen , iodine and noble gases .
In air, sulfur ignites from a temperature of around 250 ° C and burns with a blue flame, forming sulfur dioxide. The ignition point can be lowered by gases such as hydrogen sulfide and sulfur dioxide dissolved in the sulfur. In moist air, sulfur forms sulfuric acid and sulfur dioxide over time.
Sulfur does not react with non-oxidizing acids; oxidizing acids such as nitric acid oxidize sulfur to sulfate. In an alkaline solution, sulfur reacts with disproportionation to form sulfide and sulfite . In sulphidic solution, sulfur dissolves with the formation of polysulphides. In a solution containing sulfite, sulfur reacts to form thiosulfate.
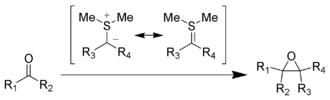
In organic chemistry, elemental sulfur is used, for example, in the Asinger reaction to prepare 3-thiazolines . The Gewald reaction - also a multi-component reaction - enables the synthesis of substituted 2-aminothiophenes, starting from elemental sulfur. Sulfur reacts with Grignard compounds to form thioethers or thiols . Cyclohexane is dehydrated to benzene with the release of hydrogen sulfide .
Isotopes
23 isotopes between 26 S and 49 S are known of sulfur, four of which are stable: 32 S (95.02%), 33 S (0.75%), 34 S (4.21%) and 36 S (0 , 02%). Of the radioactive isotopes, only the 35 S isotope has a half-life of 87.51 days, for all other isotopes the half-life is in the range of seconds or milliseconds. The 35 S isotope is created from 40 Ar by cosmic rays.
When sulphidic minerals are precipitated , there may be different isotope distributions between the solid and the mother liquor depending on the temperature and pH value. The determination of the sulfur isotope distribution in the mineral therefore allows conclusions to be drawn about the formation conditions. When sulfates are broken down by bacterial sulfate reduction, sulfur isotope fractionated. The investigation of the sulfur isotope distribution in the dissolved sulfate therefore allows conclusions to be drawn about biological reduction processes.
use
Sulfur is used both in the chemical industry and in the pharmaceutical industry , among other things for the production of sulfuric acid , dyes , pesticides and artificial fertilizers .
Production of sulfuric acid
The largest part of the sulfur, around 90%, is used to produce sulfuric acid using the contact process. In the first step, sulfur dioxide is produced by burning sulfur or roasting metal sulfides. This is converted into sulfur trioxide with air in an equilibrium reaction on a vanadium pentoxide catalyst .
About 60% of sulfuric acid is used to make fertilizers . The digestion of rock phosphate with sulfuric acid produces superphosphate , a mixture of calcium dihydrogen phosphate (Ca (H 2 PO 4 ) 2 ) and calcium sulfate (CaSO 4 · 2H 2 O).
Other fertilizers containing sulfur are ammonium sulfate and potassium sulfate . In addition, sulfuric acid is used for the digestion of ores, the production of caprolactam , as a catalyst in the alkylation of olefins, for the production of other acids such as hydrogen fluoride and in paper production using the sulfate process . Sulfuric acid is also used in numerous other processes, such as the production of phenol and acetone using the cumene hydroperoxide process .
Gaseous sulfur trioxide is widely used in the production of surfactants by sulfonating dodecylbenzene to dodecylbenzenesulfonic acid and also in the sulfation of fatty alcohols and their ethoxylates.
Vulcanization of rubber
Pure sulfur is very often used in the vulcanization of rubber . For this purpose, raw rubber is mixed with sulfur or sulfur-containing compounds, a process that was developed by Charles Goodyear in 1839 . The rubber polymer chains are crosslinked with the formation of sulfide bridges from one or more sulfur atoms, making them particularly resistant. The US vulcanizable elastomer market in 2001 was $ 5.7 billion.
Medical applications
The European Pharmacopoeia only lists sulfur for external use (Sulfur ad usum externum). The powdered sulfur forms hydrogen sulfide and other sulfides on the skin, which in turn have a bacteriostatic effect. Oral consumption also has a laxative effect. Sulfur also has a fungicidal effect and can kill parasites. Sulfur was mainly used in the past to treat acne vulgaris , scabies and superficial mycoses . They are mostly used in the form of soaps, ointments and gels.
Sulfur (abbreviation Sulph ) is one of the fourteen most important basic substances in homeopathy .
Use in the steel industry
In heavy industry, sulfur is important as an alloying element for steel. Free- cutting steels that are optimized for machining processes such as turning and drilling are often sulfur-alloyed. The addition of sulfur creates soft, line-shaped manganese sulphide inclusions in the steel, which lead to increased chip breaking.
In air, especially with increased humidity, sulfur easily develops sulfur dioxide and with further oxidation sulfuric acid. This can lead to corrosion and damage to steel structures or storage tanks.
Catalyst poison
Sulfur species in hydrocarbons are strong catalyst poisons that are effective even in low concentrations. Sulfur reacts with the catalytically active metal and other surface centers of the catalysts. The poisoning can be reversible or irreversible and the selectivity of the catalyst can be changed or deactivated altogether. Some catalysts, such as platinum - rhenium catalysts for catalytic reforming , are selectively charged with sulfur in order to influence the initial activity of the catalyst.
In older three-way catalytic converters , the sulfur stored on the catalytic converter as sulphate was released as hydrogen sulphide when operated in excess air. In vehicles with a lean-burn gasoline engine and storage catalytic converter, sulphate formation can deactivate the catalytic converter. For regeneration, the exhaust gas temperature is increased to 650 degrees Celsius and the stored sulphate is emitted as sulfur dioxide.
Others
Sulfur is used in the manufacture of black powder , as nitric sulfur in fireworks , or in other explosives .
The so-called sulfurizing is a method of preserving food, such as B. wine or dried fruit , using sulfur dioxide or sulfur salts , whereby their reductive properties ( antioxidant ) are used. Formerly that was sulfurizing wine achieved by the burning of sulfur in empty wine barrels, today is pyrosulphite added, which releases sulfur dioxide in acidic solution.
As a protective contact fungicide, sulfur acts preventively through contact with the plant surface , where it is slowly oxidized to sulfur dioxide and inhibits spore germination. The use in viticulture against the powdery mildew that grows on the leaf surface is widespread .
Ultramarine dye is obtained by burning a mixture of sulfur, kaolin , sodium sulfate , soda and activated carbon . The deep blue color of ultramarine comes from polysulphide radical ions of type • S 3 - locked in sodalite cages , which are responsible for the color in the lapis lazuli .
Another development is the sodium-sulfur cell , a rechargeable battery that is primarily used in small to medium-sized stationary battery storage power plants in Japan. The cell works at a temperature of around 300 to 350 ° C. Liquid sulfur serves as the positive electrode , a ceramic made of sodium β-aluminate (NaAl 11 O 17 ) as the solid electrolyte and liquid sodium as the negative electrode. The gross electrochemical reaction is:
Sulfur or sulfur compounds are used as lubricant additives. The drawing fats used in wire drawing mills consisted of natural fats to which sulfur bloom was added. This forms a layer of metal sulfides on the metal surface, in the case of iron, for example iron (II) sulfide (FeS), iron (II) disulfide (FeS 2 ) or iron (III) sulfide (Fe 2 S 3 ), which act as a lubricant Act. Molybdenum (IV) sulfide is known under the trade name Molykote and, due to its graphite-like structure, serves as a solid lubricant. Sulfur compounds are still added to lubricants as extreme pressure additives .
Organic chemistry
In organic chemistry, sulfur is used for the synthesis of 3-thiazolines in the Asinger reaction - a multi-component reaction.
physiology
Sulfur compounds are found in all living things and have a variety of functions. Sulfur is assimilated as sulfide or sulfate by plants and bacteria from the environment and built up into organic sulfur compounds that are ultimately ingested by animals with their food.
Occurrence and function in living things
Sulfur is contained in the proteinogenic amino acids cysteine and methionine - and in all peptides , proteins , coenzymes and prosthetic groups based on them - in the form of thiol groups (oxidation level + II) or thioether groups. Other sulfur-containing amino acids with a biological function are cystine and homocysteine . It is also contained in some cofactors ( biotin , thiamine pyrophosphate ) in a heterocyclic bond. Sulfur is therefore an essential element of living cells. Disulfide bonds are widespread and on the one hand contribute to the formation and stabilization of protein structures (e.g. in the keratin of human and animal hair and feathers), on the other hand many redox reactions in cells are based on the reversibility of this bond (in thioredoxin and glutathione ). In oxidized form, sulfur plays a biological role in the aminosulfonic acid taurine (oxidation level + IV).
The total sulfur content of the human body is around 0.25%, although the content can vary depending on the type of tissue. A total sulfur content of between 0.37% in pigs and 0.6% in humans was determined in the globins of mammals. For example, in the horn of horses, keratin consists of up to 5% sulfur.
Sulfur-containing plant substances such as the cysteine sulfoxides methiin, alliin , isoalliin and propiin can make up up to 5% of the dry matter of plants, for example in the Allium plants used as spices such as garlic and onion . In addition, secondary aroma components such as diallyl disulphide or allicin are created through enzymatic activity or oxidation in air , some of which are responsible for the typical smell and taste of these plants.
Transition metal sulfides, especially those of iron, are the active centers of a number of enzymes. The ferredoxins are iron and sulfur-containing proteins that take part in metabolic reactions as electron carriers. The ferredoxin in human metabolism is called adrenodoxin. Complex metalloenzymes such as nitrogenase , which is able to reduce elemental, molecular nitrogen (N 2 ), as well as hydrogenase and xanthine oxidase , have iron-sulfur clusters .
admission
The sulfur required for all of the substances mentioned is ingested by animals as sulfur-containing amino acids and vitamins, depending on their diet. Plants and bacteria, in turn, are able to assimilate sulfur as sulphide or sulphate and to synthesize the named amino acids and vitamins themselves.
In plant cultivation , depending on the type of plant, around 15 to 50 kg of sulfur per hectare are required in the form of sulfur-containing fertilizers. Oil plants , legumes , various fodder plants and vegetables require larger amounts of sulfur.
Sulfide oxidation
Some subgroups of the proteobacteria , collectively called the colorless sulfur-oxidizing bacteria , can oxidize sulfur compounds and sulfur and gain energy from these exergonic reactions; see for example the giant bacterium Thiomargarita namibiensis . In addition, the green sulfur bacteria are able to carry out photosynthesis by using hydrogen sulfide, sulfur or thiosulfate instead of water (H 2 O) as an electron donor for the reduction of CO 2 . This type of photosynthesis does not produce oxygen ("anoxygen"). Finally, some cyanobacteria can use this metabolic pathway. Plants and animals have enzymes in their mitochondria for the oxidation of sulfide, but these are only used to detoxify the excess cysteine and the hydrogen sulfide produced in the intestine.
Sulfur Assimilation in Plants
In vascular plants, the sulfur is absorbed as sulfate via the roots and transported via the xylem into the leaves, where most of the assimilation, coupled to photosynthesis, takes place in the chloroplasts of the mesophyll cells . The assimilation rate is only around five percent of the nitrate assimilation and one to two per thousand of the CO 2 assimilation. The basic scheme is similar to nitrate assimilation, but the energy consumption is much higher with sulfur.
The sulfate is first reduced to sulfite, which is then further reduced to hydrogen sulfide. This is bound in cysteine, the first stable compound in sulfur assimilation:
Sulphate must first be activated in the chloroplast. The enzyme sulfate adenylyl transferase forms AMP sulfate ( APS ) and pyrophosphate from sulfate and ATP . The equilibrium of this reaction is strongly on the side of ATP, the formation of APS is only possible due to the high activity of pyrophosphatase in the chloroplasts. The APS kinase then attaches another phosphate residue to the ribose residue of the APS and forms the 3-phospho-AMP sulfate ( PAPS ). The sulfate activated in this way is reduced by the PAPS reductase with the help of thioredoxin to sulfite, which is split off from the 3-phospho-AMP in the course of this reaction.
The structure of sulphite reductase is similar to nitrite reductase, it contains a siroheme and a 4-iron-4-sulfur center . With the consumption of six reduced ferredoxins it forms hydrogen sulfide. In heterotrophic tissue such as roots, the reduced ferredoxin is not provided by photosynthesis, but is reduced by NADPH .
In order to be able to bind the hydrogen sulfide to serine , it must first be activated. This is done by serine transacetylase, which transfers an acetyl residue from acetyl coenzyme A to the serine. For the formation of the acetyl-coenzyme A two energy-rich phosphates are used. The hydrogen sulfide is finally transferred to the O-acetylserine by the O-acetylserine (thiol) lyase, with cysteine and acetate being formed.
Environmental aspects
When generating energy from fossil fuels such as hard coal , lignite and crude oil , large amounts of sulfur dioxide SO 2 are released. This initially remains in the earth's atmosphere as a gas or dissolved in the water of the clouds . It also forms part of the harmful smog . It can be broken down by being oxidized by oxygen to sulfur trioxide SO 3 and being washed out as sulfuric acid H 2 SO 4 by the rain. This creates another problem, as it contributes to the acidification of the soil as part of the acid rain .
For this reason, measures for flue gas desulfurization (FGD) have been required by law in Germany since the 1970s . This is usually done by washing lime. The flue gases are sprayed with calcium hydroxide solution in an absorber, which converts the sulfur dioxide into calcium sulfate (gypsum) with further oxidation.
In addition, the desulfurization of vehicle fuels has been promoted for several years . With these regulations and their implementation, sulfur (dioxide) emissions have been drastically reduced since the 1960s.
Natural gas and coke oven gas contain varying amounts of hydrogen sulfide from the source. Low-boiling gasoline naturally contains virtually no sulfur. Higher-boiling petroleum fractions such as diesel and heavier oils typically contain more sulfur, such as thiols . Extra light heating oil , which is burned in apartments, and diesel fuel , which is also used for civil purposes and on land, were reduced in sulfur content in Europe from around 1999. Heating and power plants convert large amounts of heavy fuel oil with a comparatively high sulfur content on land and are desulphurised in the flue gas for technical reasons. Since marine diesel engines, which also burn heavy fuel oil, tend to emit far away from land at sea, emission limits for sulfur (SO 2 ) were only prescribed here late, from 2012 ; Here too, desulphurisation is only carried out in the exhaust gas.
Cleaner processes have made the production of sulphite cellulose in paper mills and viscose fiber less odorous. In the 1970s, a kilometer-wide smell from the high chimney of the man-made fiber factory in Lenzing was still common and served residents of the Attersee as a good-weather wind direction indicator. Odor carriers here are thiols and hydrogen sulfide.
The International Maritime Organization (IMO) has set the limit values for ship exhaust gases from 4.5 percent sulfur to 3.5 percent from 2012 and 0.5 percent from 2020. For the North and Baltic Seas, a sulfur content in the exhaust gases of 0.1 percent was set from 2015.
In Glencore's Mopani Copper Mines , the situation is still dramatic (as of June 2019).
proof
There are various detection reactions for sulfur.
- Sulfur compounds in after reduction by elemental sodium in sodium sulfide transferred. Sulphide anions are detected with lead (II) salt solutions, whereby a black precipitate of lead (II) sulphide is formed:
- When solid, i.e. undissolved sulfides are acidified, a characteristic odor of rotten eggs is created (H2S gas displacement reaction ). The gas blackens lead acetate paper .
- Oxidation of sulfur-containing compounds produces sulfite and sulfate . The latter is detected with barium (II) salt solutions. A white precipitate of barium sulfate is formed:
- Sulphite is detected with potassium hydrogen sulphate. When the substance to be tested for sulfite is rubbed with potassium hydrogen sulfate, the pungent-smelling sulfur dioxide is produced . The following reaction equation results for sodium sulfite :
- The Wickbold method is suitable for the quantitative determination of small amounts of sulfur .
- In nuclear magnetic resonance spectroscopy , the 33 S nucleus is used to detect sulfur in the form of sulfates and sulfites. The nucleus shows only low sensitivity and low natural occurrence.
- In gas chromatography, sulfur can be determined selectively by combining it with chemiluminescence or plasma emission detectors. To determine the total sulfur content of organic compounds, these can first be catalytically converted into hydrogen sulfide, which is detected by flame photometry.
links
In compounds, sulfur occurs in all oxidation states between −II ( sulfides ) and + VI ( sulfates , sulfur trioxide and sulfuric acid ).
Hydrogen compounds
Hydrogen sulfide (H 2 S) is a colorless, poisonous gas that smells like rotten eggs in low concentrations and is produced by the reaction of sulfides (M x S y ) with strong acids, for example hydrochloric acid (HCl). It occurs as a natural companion to natural gas and is produced in large quantities during the hydrodesulphurisation of petroleum fractions. Hydrogen sulfide is a weak acid. It is flammable, colorless and not very soluble in water and somewhat more soluble in alcohol. Hydrogen sulfide and metal oxides or hydroxides form sulfides such as cinnabar (HgS) and lead sulfide (PbS). The poor solubility of heavy metal sulfides is used in analytical chemistry in the separation process to precipitate the metals of the hydrogen sulfide group .
Disulfan (H 2 S 2 ) is an inconsistent liquid. However, it forms many salts such as pyrite . Their salts ( disulfides ) contain the anion S 2 2− . Disulfan is the first member of the homologous series of polysulfanes.
Oxides
Sulfur dioxide is the anhydride of sulphurous acid and a colorless, mucous membrane-irritating, pungent smelling and sour-tasting, poisonous gas. It is very soluble in water and forms sulphurous acid to a very small extent with water.
Sulfur trioxide is the anhydride of sulfuric acid. Under normal conditions it forms colorless, needle-shaped crystals that are extremely hygroscopic and react very violently with water. Sulfur trioxide boils at 44.45 ° C.
Sulfur monoxide is only stable in diluted form. In concentrated form, it quickly converts to disulfur dioxide. It was detected in interstellar space.
Oxo acids and salts
Sulfur forms a number of oxo acids , of which sulfuric acid has by far the greatest technical importance. The oxidation states that occur range from + VI (sulfuric acid) to almost 0 (oligosulfanedisulfonic acids, HSO 3 S x SO 3 H). The acids cannot all be isolated in their pure form, but they do form a number of salts and their hydrido isomers. Sulphurous acid cannot be isolated as a pure substance, whereas sulphite and hydrogen sulphite salts are known to be stable compounds.
| Acids of the type H 2 SO n | ||||
|---|---|---|---|---|
| Oxidation level of sulfur |
structure | Acids | Salts | |
| + II |
Sulfoxylic acid H 2 SO 2 |
Sulfoxylates | ||
| + IV |
Sulphurous acid H 2 SO 3 |
Sulfites | ||
| + VI |
Sulfuric acid H 2 SO 4 |
Sulfates | ||
| + VI |
Peroxo (mono) sulfuric acid H 2 SO 5 |
Peroxosulfates | ||
| Acids of the type H 2 S 2 O n | ||||
|---|---|---|---|---|
| Medium oxidation state of sulfur |
structure | Acids | Salts | |
| + I |
Thiosulphurous acid H 2 S 2 O 2 |
Thiosulfites (unknown) |
||
| + II |
Thiosulfuric acid H 2 S 2 O 3 |
Thiosulfates | ||
| + III |
Dithionic acid H 2 S 2 O 4 |
Dithionite | ||
| + IV |
Disulfurous acid H 2 S 2 O 5 |
Disulfites | ||
| + V |
Dithionic acid H 2 S 2 O 6 |
Dithionates | ||
| + VI |
Disulfuric acid H 2 S 2 O 7 |
Disulfates | ||
| + VI |
Peroxodisulfuric acid H 2 S 2 O 8 |
Peroxodisulfates | ||
Nitrogen compounds
Tetrasulphur tetranitride S 4 N 4 is a gold-red solid that serves as the starting compound for various sulfur-nitrogen compounds.
Disulfur dinitride S 2 N 2 is in the form of a four-membered, rectangular-planar ring. The compound can be obtained by reacting tetrasulfur tetranitride with silver .
Polythiazyl (SN) x was the first inorganic polymer with electrical conductivity. At very low temperatures below 0.26 K, the material is superconducting. Polythiazyl is obtained from disulfur dinitride.
Sulfur nitrogen (SN) has been detected as a component of intergalactic molecular clouds. In the laboratory it can be obtained by electrical discharges in a nitrogen-sulfur gas.
Halogen compounds
Sulfur halides of the type SX n ( n = 2, 4) are known from fluorine and chlorine , fluorine also forms a hexafluoride. A number of mixed halogen compounds are also known. Oxygen-halogen compounds of the type SOX 2 (thionyl halides), SO 2 X 2 (sulfuryl halides ) and SOX 4 are known. Only one iodopolysulfane compound of the I 2 S n type is known of iodine .
Sulfur fluorides
Sulfur hexafluoride (SF 6 ) is a colorless, odorless, non-toxic gas that is non-flammable and extremely inert. Among other things, it is used as an insulating gas in medium and high voltage technology. The gas is used as a tracer for the detection of wind currents and for odor spread studies. However , its use is controversial because of the high global warming potential .
Disulfur decafluoride (S 2 F 10 ) is a colorless liquid with a smell of sulfur dioxide.
Sulfur tetrafluoride (SF 4 ) is a colorless, non-flammable gas with a pungent odor. It decomposes in water to form hydrogen fluoride . It acts as a weak Lewis acid and forms, for example, 1: 1 adducts with organic bases such as pyridine and triethylamine .
Sulfur difluoride (SF 2 ) is a colorless gas that quickly dimerizes to disulfur difluoride (S 2 F 2 ). The latter is in the form of two gaseous isomers, thiothionyl fluoride (S = SF 2 ) and difluorodisulfane (FSSF).
Sulfur chlorides
Disulfur dichloride (S 2 Cl 2 ) is obtained by chlorinating elemental sulfur. Disulfur dichloride is used in the production of rubber vulcanizing agents and other organic sulfur compounds. It acts as a catalyst in the chlorination of acetic acid .
Sulfur dichloride (SCl 2 ), a deep red liquid, is produced by reacting disulfur dichloride with chlorine gas . It is dissolved in carbon disulfide (CS 2 ) and used for the cold vulcanization of rubber . During the First World War, sulfur dichloride was used to manufacture the warfare agent S-mustard .
Sulfur tetrachloride (SCl 4 ) is produced by the direct chlorination of sulfur with chlorine . It is stable in the solid state and below −30 ° C; above it it decomposes, producing chlorine and sulfur dichloride .
Mixed sulfur halides and oxohalides
Sulfur pentafluorochloride (SF 5 Cl), a colorless gas, is used in preparative chemistry to represent organic components with carbon-sulfur double and triple bonds.
Sulfur pentafluorobromide (SF 5 Br), a colorless gas, can be produced from sulfur tetrafluoride, silver (II) fluoride and elemental bromine.
The thionyl halides OSX 2 are known from fluorine, chlorine and bromine, the sulfuryl halides from fluorine and chlorine.
Organic compounds
Organosulfur compounds are organic compounds that contain sulfur. The structure, the occurrence and the applications of the organosulfur compounds are diverse. Many natural substances , including two essential amino acids, are organic sulfur compounds. Organic sulfur compounds occur in fossil fuels, for example in the form of thiols or thioethers . Anionic surfactants are sodium or ammonium salts of sulfonic acids or sulfuric acid half-esters . In flotation technology , certain sulfur compounds such as xanthates , dithiophosphoric acid esters , mercaptans or alkyl sulfonates are suitable as so-called collectors.
The carbon-sulfur single bond is both longer and weaker than the carbon-carbon bond. The bond lengths are between 183 pm in methanethiol and 174 pm in thiophene . The dissociation energy of the carbon-sulfur bond for thiomethane is 312.5 kJ / mol, the dissociation energy for dimethyl sulfide and dimethyl ether is 305 and 322 kJ / mol.
The organic chemistry of sulfur is diverse. The organic sulfur analogues exist for practically all carbon-oxygen compounds. Their reactions often differ considerably from oxygen compounds.
Well-known organosulfur compounds are thiols , which are also called mercaptans. Thiols are formed, for example, from the reaction of potassium hydrogen sulfide with alkyl halides. The alkylation of thiourea with alkyl halides and subsequent reaction with sodium hydroxide solution also leads to thiols and the release of urea. Thiols are part of many natural substances such as the defensive substances of the skunk (3-methylbutanethiol) and often have an unpleasant odor. They can easily be converted into disulfides by oxidation or into sulfonic acids via the sulfenic and sulphinic acids . Disulfide bridges stabilize the structure of proteins and peptide hormones such as insulin . When laying a permanent wave , the cystine bonds in the keratin are broken by reduction with thioglycolic acid . Then the hair is brought into the desired shape. Subsequent oxidation of the thiol groups in the keratin with hydrogen peroxide to disulfide bridges fixes the hair in this new shape. Garlic , leeks and other plants contain a number of organosulfur active ingredients such as alliin , which have antibiotic properties.
Thioethers can be prepared, for example, by the reaction of alkali sulfide with alkyl halides or by the Pummerer rearrangement . Trialkylsulfonium salts are formed with excess alkyl halide. Thioethers can easily be oxidized to sulfoxides and sulfones . Sulfoxides with two different alkyl radicals are chiral on the sulfur atom. The lone pair of electrons acts as the fourth substituent.
As a heterocyclic compound , for example, thiophene is known. Carbon-sulfur-oxygen compounds such as sulfoxides , which are used as solvents like dimethyl sulfoxide , are common. Sulphonic acids or their salts, the sulphonates , are used as surfactants . In organic synthesis, thioacetals are used as synthons for umpolung the carbonyl function, for example in the Corey-Seebach reaction . In the Johnson-Corey-Chaykovsky reaction, a carbonyl function is converted into an epoxide using sulfur ylides .
A large number of organoleptic substances containing sulfur are known to the chemistry of fragrances and flavors . She has identified a number of substances from natural sources and uses the hetero element to design new fragrances and to determine olfactory structure-effect relationships . The lowest odor threshold (10 −4 ppb ) that has ever been measured in natural aromas comes from thioterpineol , the sulfur analogue of α- terpineol, isolated from grapefruit . The structurally similar 8-thio- p- menth-3-one with the typical smell of black currant has slightly weaker potency . These substances are surpassed by thiamine - photolyte bis (2-methyl-3-furyl) disulfide, which is one of the strongest odor compounds in organic chemistry. In galban resin , thioesters with a pronounced smell are found as structural relatives of the senecioesters . A monoterpenoid thiophene analogous to perillen is contained in hops . Shiitake contains the aromatic substance 1,2,3,5,6-pentathiepan (lenthionine). Asparagus contains 1,2-dithiolanes. Radish and radish put the 4-methylsulfinyl-3-butenyl isothiocyanate free.
The colorless and odorless natural gas is odorized with tetrahydrothiophene in order to guarantee a slight odor perception in the event of a leak. Escaping natural gas can therefore be detected in the event of the smallest leak.
Sulfur-containing ligands
As a ligand in organometallic chemistry, sulfur has a wide range of coordination options. The metal-sulfur complexes are considered model compounds for the study of metalloenzymes . Sulfur occurs in the complexes as a bridging mono-, di- and polysulfide ligand, as a sulfide, as a sulfur ring of various sizes or as η 2 -disulfide.
literature
- Ralf Steudel , Hans-Joachim Mäusle: Liquid sulfur - a raw material with a complicated composition. In: Chemistry in Our Time. 14th year 1980, No. 3, pp. 73-81, doi: 10.1002 / ciuz.19800140302 .
- Ralf Steudel (Ed.): Elemental Sulfur and Sulfur-Rich Compounds (part I & II). In: Topics in Current Chemistry. Volume 230 & 231, Springer, Berlin 2003.
- Harry H. Binder: Lexicon of the chemical elements - the periodic table in facts, figures and data. S. Hirzel Verlag, Stuttgart 1999, ISBN 3-7776-0736-3 .
- Winnacker-Küchler: Chemical Technology: Processes and Products. Volume 3: Inorganic Basic Materials, Intermediate Products. edited by Roland Dittmeyer, Wilhelm Keim , Gerhard Kreysa, Alfred Oberholz. Wiley-VCH Verlag, 2005, ISBN 3-527-30768-0 .
- Max Schmidt : Sulfur - what is it actually? In: Chemistry in Our Time. 7, 1973, pp. 11-18, doi: 10.1002 / ciuz.19730070103 .
- Max Schmidt: Elemental sulfur - a current problem in theory and practice. In: Angewandte Chemie. 85, 1973, pp. 474-484, doi: 10.1002 / anie.19730851103 .
- Joachim Schroeter: The sulfur in medicine and in older chemistry. In: Ciba Zeitschrift 9, 1945, No. 98 ( Der Schwefel ), pp. 3497-3502.
Web links
Individual evidence
- ↑ a b Harry H. Binder: Lexicon of the chemical elements. S. Hirzel Verlag, Stuttgart 1999, ISBN 3-7776-0736-3 .
- ↑ The values for the properties (info box) are taken from www.webelements.com (sulfur) , unless otherwise stated .
- ↑ The standard value recommended by IUPAC is given, since the isotopic composition of this element can vary locally, the mass range given in brackets results for the mean atomic weight. See: Michael E. Wieser, Tyler B. Coplen: Atomic weights of the elements 2009 (IUPAC Technical Report). In: Pure Appl. Chem. 2011, p. 1, doi: 10.1351 / PAC-REP-10-09-14 .
- ↑ CIAAW, Standard Atomic Weights Revised 2013 .
- ^ IUPAC, Standard Atomic Weights Revised 2013 (Excel table) .
- ↑ a b c d e f Entry on sulfur in Kramida, A., Ralchenko, Yu., Reader, J. and NIST ASD Team (2019): NIST Atomic Spectra Database (ver. 5.7.1) . Ed .: NIST , Gaithersburg, MD. doi : 10.18434 / T4W30F ( https://physics.nist.gov/asd ). Retrieved June 11, 2020.
- ↑ a b c d e f Entry on sulfur at WebElements, https://www.webelements.com , accessed on June 11, 2020.
- ^ SJ Rettig, J. Trotter: Refinement of the Structure of Orthorhombic Sulfur, α-S 8 . In: Acta Crystallographica Section C. Volume 43, 1987, pp. 2260-2262, doi: 10.1107 / S0108270187088152 .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Properties of the Elements and Inorganic Compounds, pp. 4-142-4-147. The values there are based on g / mol and are given in cgs units. The value specified here is the SI value calculated from it, without a unit of measure.
- ↑ a b Yiming Zhang, Julian RG Evans, Shoufeng Yang: Corrected Values for Boiling Points and Enthalpies of Vaporization of Elements in Handbooks. In: Journal of Chemical & Engineering Data. 56, 2011, pp. 328-337, doi: 10.1021 / je1011086 .
- ^ Entry on sulfur in the GESTIS substance database of the IFA , accessed on August 9, 2016(JavaScript required) .
- ↑ Entry on Sulfur in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Etymological dictionary of the German language . 1967, p. 690.
- ↑ a b N. Figurowski: The discovery of the chemical elements and the origin of their names. Aulis-Verlag Deubner, Cologne 1981, ISBN 3-7614-0561-8 , pp. 179-180.
- ↑ George Rapp: Archaeomineralogy. 2nd Edition. Springer, 2009, ISBN 978-3-540-78593-4 , p. 242.
- ↑ Odyssey. book 22, lines 480–495.
- ^ John F. Healy: Pliny the Elder on science and technology. Oxford University Press, 1999, ISBN 0-19-814687-6 , pp. 247-249.
- ↑ Pliny the Elder: Naturalis historia. Translated from the Latin by John Bostock & al. tape 35 , chap. 15 (English, full text ).
- ^ Fritz Seel : History and chemistry of black powder. Le charbon fait la poudre. In: Chemistry in Our Time . 22, 1988, pp. 9-16, doi: 10.1002 / ciuz.19880220103 .
- ^ Franz Maria Feldhaus : Berthold (inventor of gunpowder) . In: Allgemeine Deutsche Biographie (ADB). Volume 55, Duncker & Humblot, Leipzig 1910, pp. 617-619.
- ↑ Gundolf Keil : The "Cirurgia" Peters von Ulm. Investigations into a memorial of old German specialist prose with a critical edition of the text. (= Research on the history of the city of Ulm. Volume 2). Stadtarchiv, Ulm 1961 (also philosophical dissertation Heidelberg 1960), p. 471.
- ^ William H. Brock: Viewegs Geschichte der Chemie . Springer, 1997, ISBN 3-540-67033-5 , pp. 67 ( limited preview in Google Book search).
- ↑ J. Gay Lussax, LJ Thenard: testing the decomposing investigations of Mr. Davy about the nature of sulfur and phosphorus.. In: Annals of Physics . Volume 35, Issue 7, 1810, pp. 292-310, doi: 10.1002 / andp.18100350704 .
- ↑ Peter Kurzweil, Paul Scheipers: Chemistry: Fundamentals, structural knowledge, applications and experiments. Vieweg + Teubner; (2011), S., ISBN 3-8348-1555-1 .
- ^ WC Zeise: Jahresber. Progress Chem. 3, 1824, p. 80; 16, 1837, p. 302.
- ^ Lawrie Lloyd: Handbook of Industrial Catalysts (Fundamental and Applied Catalysis). Springer US, 2011, ISBN 978-0-387-24682-6 , p. 29.
- ↑ a b Max Schmidt: Sulfur - what is it actually? In: Chemistry in Our Time. Volume 7, Issue 1, February 1973, pp. 11-18, doi: 10.1002 / ciuz.19730070103 .
- ^ Ernst Beckmann: Cryoscopic determinations in iodine. In: Journal of Inorganic Chemistry . Volume 77, Issue 1, pp. 200-208, October 3, 1912, doi: 10.1002 / zaac.19120770115 .
- ^ BE Warren, JT Burwell: The Structure of Rhombic Sulfur. ( Memento of October 15, 2012 in the Internet Archive ) In: J. Chem. Phys. 3, 6, 1935, doi: 10.1063 / 1.1749557 .
- ^ Sulfur. Retrieved July 3, 2012 .
- ^ Mineralienatlas: Mineralienportrait Schwefel
- ^ IMA / CNMNC List of Mineral Names. (PDF; 1.9 MB) Retrieved July 3, 2012 . (English, PDF 1.8 MB, p. 272).
- ^ A b Hugo Strunz , Ernest H. Nickel: Strunz Mineralogical Tables . 9th edition. E. Schweizerbart'sche Verlagbuchhandlung (Nägele and Obermiller), Stuttgart 2001, ISBN 3-510-65188-X , p. 53 .
- ↑ a b Sulfur. (PDF; 447 kB) Retrieved July 3, 2012 . (English, PDF 436.8 kB).
- ↑ Mineral Species containing Sulfur (S). Retrieved July 3, 2012 .
- ↑ Peter Adolphi, Bernd Ullrich: Fixation and reactivity of sulfur in brown coal. In: Proc. XXXII. Power Plant Technology Colloquium, Use of Difficult Fuels in Power Plants, Dresden October 24 and 25, 2000. pp. 109–116.
- ^ The chemical composition of seawater. Retrieved July 3, 2012 .
- ↑ An unusual enzyme enables methane to be inactivated. (PDF; 343 kB) Retrieved July 3, 2012 .
- ↑ Water dictionary: Sulphate. Retrieved July 5, 2012 .
- ^ Atmospheric Aerosols: What Are They, and Why Are They So Important? Retrieved July 2, 2012 .
- ^ A b Alan R. Wellburn, U. Gramm (trans.), D. Mennecke-Bühler (trans.): Air pollution and climate change: Effects on flora, fauna and humans. Springer, 1997, ISBN 3-540-61831-7 , p. 31 and 104 ( limited preview in Google Book search).
- ↑ Niemeier, U., & Tilmes, S. (2017). Sulfur injections for a cooler planet. Science, 357 (6348): 246-248. doi: 10.1126 / science.aan3317
- ↑ Climate manipulation: With technology against global warming. Berliner Zeitung, August 20, 2018, accessed on December 14, 2019 .
- ↑ Tilmes, S., Muller, R., & Salawitch, R. (2008). The sensitivity of polar ozone depletion to proposed geoengineering schemes. Science, 320 (5880), 1201-1204. doi: 10.1126 / science.1153966
- ↑ Researchers warn against geoengineering. Heise Online, April 24, 2008, accessed December 14, 2019 .
- ↑ Thomas Leustek, Kazuki Saito: Sulfate Transport and Assimilation in Plants. In: Plant Physiology . Volume 120, No. 3, July 1999, pp. 637-644, doi: 10.1104 / pp.120.3.637 .
- ↑ Daniel C. Boice, Celine Reyle: The Nature of Diatomic Sulfur in Comets. In: Formation of Cometary Material, 25th meeting of the IAU, Joint Discussion 14, July 22, 2003, Sydney, Australia. bibcode : 2003IAUJD..14E..38B .
- ^ Volcanism on Io. Retrieved July 3, 2012 .
- ↑ Swimming a Salty Sea. Retrieved July 3, 2012 .
- ↑ CA Gottlieb, EW Gottlieb, MM Litvak, JA Ball, H. Pennfield: Observations of interstellar sulfur monoxide. In: Astrophysical Journal. 219, 1, 1978, pp. 77-94. bibcode : 1978ApJ ... 219 ... 77G .
- ↑ MW Sinclair, N. Fourikis, JC Ribes, BJ Robinson, RD Brown, PD Godfrey: Detection of interstellar thioformaldehyde. In: Australian Journal of Physics. Volume 26, p. 85. bibcode : 1973AuJPh..26 ... 85S .
- ^ To Find Alien Volcanic Eruptions, Look for Foul Gas. Retrieved July 3, 2012 .
- ↑ An Introduction to Sulfur. Retrieved July 3, 2012 .
- ^ Sulfur. (PDF; 85 kB) Retrieved July 3, 2012 .
- ^ Emil Raymond Riegel, James Albert Kent: Kent and Riegel's handbook of industrial chemistry and biotechnology. Volume 1, p. 1162 ( limited preview in Google Book search).
- ↑ The processing of natural gas. (PDF; 816 kB) Retrieved July 5, 2012 .
- ↑ Sulfur - a natural but undesirable accompanying substance. Archived from the original on November 29, 2011 ; Retrieved July 3, 2012 .
- ↑ Roland Dittmeyer, Wilhelm Keim , Gerhard Kreysa, Alfred Oberholz (eds.): Winnacker / Küchler: Chemical technology: processes and products. Volume 3: Inorganic Basic Materials, Intermediate Products. Verlag Wiley-VCH, Weinheim 2005, ISBN 3-527-30768-0 .
- ↑ Ursula Klein: Connection and affinity: The foundation of modern chemistry at the turn of the 17th to the 18th century. Birkhäuser Verlag, 1994, ISBN 3-7643-5003-2 ( limited preview in the Google book search).
- ^ R. Dittmeyer, W. Keim, G. Kreysa, A. Oberholz: Winnacker · Küchler: Chemische Technik. Volume 3: Inorganic Basic Materials, Intermediate Products. 5th edition. Wiley-VCH, Weinheim 2005, ISBN 3-527-30768-0 , pp. 33–34 ( full text ; PDF; 7.9 MB).
- ↑ Liquid sulfur on the rails. (PDF; 2.4 MB) In: Cargo Magazin 2/2005, p. 17 , archived from the original on March 9, 2014 ; Retrieved July 11, 2012 .
- ↑ Rosickýite. (PDF; 61 kB) Accessed July 4, 2012 .
- ^ Paul D. Bartlett and William R. Roderick: Hexaatomic sulfur . In: Henry F. Holtzclaw, Jr. (Ed.): Inorganic Syntheses . tape 8 . McGraw-Hill Book Company, Inc., 1966, pp. 100-103 (English).
- ↑ R. Engel: Compt. Rend., 112, 866 (1891).
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 552.
- ^ A b A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , p. 549.
- ↑ F. Asinger , M. Thiel: Simple syntheses and chemical behavior of new heterocyclic ring systems. In: Angewandte Chemie . 70, 1958, pp. 667-683, doi: 10.1002 / anie.19580702202 .
- ↑ a b Jerry March: Advanced Organic Chemistry. 3. Edition. Wiley & Sons Verlag, 1985, ISBN 0-471-60180-2 , pp. 550 and 1053.
- ↑ G. Audi, FG Kondev, Meng Wang, WJ Huang, S. Naimi: The NUBASE2016 evaluation of nuclear properties. In: Chinese Physics C. 41, 2017, S. 030001, doi: 10.1088 / 1674-1137 / 41/3/030001 ( full text ).
- ↑ Sulfur isotope investigations at the lead-zinc ore deposit in Grund (West Harz, Federal Republic of Germany) Abstract
- ↑ Evidence of reductive processes in acidic old dumps from lignite mining. (PDF; 198 kB) Accessed July 4, 2012 .
- ^ The History of the Contact Sulfuric Acid Process. (PDF; 157 kB) Accessed July 1, 2012 .
- ^ UN Industrial Development Organization: Fertilizer Manual. Verlag Springer Netherlands, 1998, ISBN 0-7923-5032-4 .
- ↑ Tharwat F. Tadros: Applied Surfactants: Principles and Applications. Wiley-VCH Verlag, 2005, ISBN 3-527-30629-3 .
- ↑ Synthetic Rubber (Vulcanizable Elastomers). Retrieved July 4, 2012 .
- ↑ Pharmacopoea Europaea , 6th edition, basic work 2008, monograph 6.0 / 0953.
- ↑ Heinrich Buess : Historical facts on sulfur therapy. In: Ciba magazine. 9, 1945, No. 98 ( Der Schwefel ), pp. 3516-3525.
- ↑ Pharmacopoeia Commentary. Scientific explanations on the European Pharmacopoeia and the German Pharmacopoeia. Wissenschaftliche Verlagsgesellschaft, Stuttgart 2004, ISBN 3-8047-2575-9 , monograph sulfur for external use. 23rd delivery, 2006.
- ↑ W. Sexton, H. Schlerkmann, G. Schmitt, W. Panning, D. Steinmetz: corrosion reactions of elemental sulfur with carbon steel in aqueous media. In: Materials and Corrosion / Werkstoffe und Korrosion. 35, 1984, pp. 556-565, doi: 10.1002 / maco.19840351204 .
- ↑ Sulfur as a Catalyst Poison (PDF; 52 kB).
- ↑ Why do some automotive catalytic converters smell like rotten eggs?
- ↑ Use of pesticides and biocidal products in non-agriculture and forestry areas. (PDF; 482 kB) Accessed July 4, 2012 .
- ↑ Three thousand years of ultramarine. Archived from the original on February 2, 2012 ; Retrieved July 4, 2012 .
- ↑ Additives. Archived from the original on November 20, 2012 ; Retrieved July 4, 2012 .
- ↑ Friedrich Asinger : About the joint effect of sulfur and ammonia on ketones. In: Angewandte Chemie. 68, 1956, p. 413.
- ^ Total Sulfur, Cystine, and Methione Content of blood globins of mammalian species. (PDF; 279 kB) Accessed July 4, 2012 .
- ↑ Feeding the hoof - biochemical principles and examples for optimization. (PDF; 116 kB) Accessed July 4, 2012 .
- ↑ What is the sulfur doing in the onion? Retrieved July 5, 2012 .
- ↑ The kitchen onion from a chemical point of view. Archived from the original on January 18, 2012 ; Retrieved July 5, 2012 .
- ↑ How much sulfur do plants need? Retrieved July 5, 2012 .
- ^ Joseph W. Lengeler, G. Drews, Hans Günter Schlegel: Biology of the prokaryotes . Thieme, Stuttgart 1999, ISBN 3-13-108411-1 , p. 245-251, 330-336 .
- ↑ MH Stipanuk, I. Ueki: Dealing with methionine / homocysteine sulfur: cysteine metabolism to taurine and inorganic sulfur. In: Journal of inherited metabolic disease. Volume 34, Number 1, February 2011, pp. 17-32. doi: 10.1007 / s10545-009-9006-9 . PMID 20162368 . PMC 290177 (free full text). (Review).
- ↑ a b c d e Hans-Walter Heldt : Plant biochemistry . Spektrum Akademischer Verlag, Heidelberg 1996, ISBN 3-8274-0103-8 , pp. 321-333.
- ↑ Big pots become eco. Archived from the original on September 6, 2010 ; Retrieved July 5, 2012 .
- ↑ Res Gehriger: Samples in Zambia - Sulfur gases from Glencore's copper works endanger local residents. In: srf.ch . June 12, 2019, accessed June 13, 2019 .
- ↑ 33 Sulfur NMR. Retrieved July 5, 2012 .
- ↑ T. Ubuka, T. Abe, R. Kajikawa, K. Morino: Determination of hydrogen sulfide and acid-labile sulfur in animal tissues by gas chromatography and ion chromatography. In: Journal of Chromatography B: Biomedical Sciences and Applications . 757, No. 1, 2001, pp. 31-37, doi: 10.1016 / S0378-4347 (01) 00046-9 .
- ↑ disulfane (H 2 S 2 ). Retrieved July 5, 2012 .
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , p. 578.
- ^ Peter Paetzold : Chemistry: An introduction. P. 366 ( limited preview in Google Book search).
- ^ NN Greenwood, A. Earnshaw: Chemical Elements. 2nd Edition. Butterworth-Heinemann, Boston, MA 1997, pp. 721-725.
- ↑ T. Chivers: Guide To Chalcogen-Nitrogen Chemistry. World Scientific Publishing Company, Singapore 2004, ISBN 981-256-095-5 .
- ↑ Hans P. Latscha, Helmut A. Klein: Inorganische Chemie: Chemie-Basiswissen I. Volume 1, P. 374 ( limited preview in the Google book search).
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , p. 580.
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , p. 553.
- ↑ Information acquisition and database. (PDF; 11.8 MB) In: Urban Climate Manual. 2010, pp. 65ff , accessed on July 5, 2012 .
- ↑ Entry on disulfur decafluoride in the GESTIS substance database of the IFA , accessed on December 13, 2019 (JavaScript required)
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , p. 564.
- ↑ G. Brauer (Ed.): Handbook of Preparative Inorganic Chemistry. 2nd Edition. Volume 1, Academic Press, 1963, pp. 371-372.
- ↑ Konrad Seppelt: Sulfur / carbon double and triple bonds (PDF; 77 kB), In: Pure & Appl. Chem. Vol. 59, No. 8, 1987, pp. 1057-1062.
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , p. 590.
- ↑ L. Pauling: The nature of the chemical bond. Verlag Chemie, Weinheim 1973, ISBN 3-527-25217-7 , p. 286.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Molecular Structure and Spectroscopy, pp. 9-77.
- ↑ Why would you want to plug protein into the socket? Retrieved July 5, 2012 .
- ^ Günther Ohloff : Fragrances and olfactory sense - The molecular world of fragrances ; Springer Verlag, ISBN 978-3-540-52560-8 .
- ↑ Edouard Demole, Paul Enggist, Günther Ohloff: 1-p-menthenes-8-thiol: A Powerful Flavor Impact constituent of grapefruit juice (Citrus parodisi Macfayden). In: Helv. Chem. Acta. 65, 1982, pp. 1785-1794; doi: 10.1002 / hlca.19820650614 .
- ↑ Ohloff, p. 13.
- ↑ Ohloff, p. 60.
- ↑ Ohloff, p. 176.
- ↑ Safety data sheet for natural gas, dried (according to DVGW worksheet G 260, 2nd gas family). Archived from the original on September 15, 2016 ; Retrieved July 5, 2012 .
- ↑ Joachim Wachter: Synthesis, structure and reactivity of sulfur-rich cyclopentadienyl transition metal complexes: Sulfur chemistry from an organometallic point of view. In: Angewandte Chemie . Volume 101, Issue 12, 1989, pp. 1645-1658.
























![{\ mathrm {S_ {8} +5 \ SbF_ {5} \ longrightarrow S_ {8} ^ {{2 +}} + 2 \ [Sb_ {2} F _ {{11}}] ^ {-} + SbF_ { 3}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/e0cf944b53a4658115c3528b4945d7e5b24f6146)