Chemiluminescence
The phenomenon known as chemiluminescence ( English : chemiluminescence ) or chemiluminescence is a process in which electromagnetic radiation in the range of ultraviolet and visible light is emitted through a chemical reaction . If the luminescence takes place in the infrared range, the term infrared chemiluminescence is used.
The Bioluminescence is a special case of chemiluminescence in biological systems, such as fireflies .
discovery
Already in 1000 BC The first theses about bioluminescent organisms were described in China. The first chemiluminescence reaction was discovered by accident in 1669 by the Hamburg alchemist Heinrich Hennig Brand ( Phosphorus Mirabilis , glow of phosphorus fumes when oxidized by atmospheric oxygen or water). He suspected that there was gold in human urine , whereupon he evaporated a few thousand liters and reduced it with coal . The phosphate present in the urine was reduced to phosphorus and then made to glow by subsequent oxidation. Eilhard Wiedemann coined the term chemiluminescence in 1888 .
basis

The emission of light in chemiluminescence is a consequence of the transition of an electron from an excited state to an energetically lower state, possibly the ground state . In contrast to fluorescence or phosphorescence , this excited state is achieved in chemiluminescence through a chemical reaction.
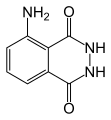
The starting materials for chemiluminescence reactions are called luminophores . The best known is 3-aminophthalic acid hydrazide ( luminol ). Under peroxyoxalate is meant reactions of hydrogen peroxide with derivatives of oxalic acid in the presence of sensitizers. Many luminophores are 1,2- or 1,4-dicarbonyl compounds such as oxalic acid esters (e.g. diphenyl oxalate and its derivatives ) and luminol .
Diphenyl oxalate , a 1,2-dicarbonyl compound
3-aminophthalic hydrazide ( luminol ), a 1,4-dicarbonyl compound
Most chemiluminescence reactions initially produce an unstable intermediate with a peroxide bridge, the energy-rich decay product of which forms a charge transfer complex with a dye molecule, the sensitizer , and energy at the same time as the sensitizer ( English sensitizer ; a molecule with several double or triple bonds ) this gives off. The excited dye molecule then sends out a light quantum whose wavelength depends on the structure of the dye used.
Under certain conditions, an oscillating chemiluminescence can also be generated, e.g. B. by combining the Orban oscillator with luminol.
Applications
Probably the best known chemiluminescence reaction is the oxidation of luminol by hydrogen peroxide in the presence of iron or manganese ions , which is used in forensics to make traces of blood visible (the blood pigment hemoglobin contains Fe 2+ ions). The oxidized luminol molecule also serves as a sensitizer.
In gas analysis, chemiluminescence is used in the oxidation of nitrogen monoxide to nitrogen dioxide in order to detect nitrogen monoxide. If nitrogen dioxide is catalytically reduced in the analyzer before oxidation, both nitrogen oxides can be detected.
Plastic tubes are commercially available that emit intense, long-lasting light when they are bent ( glow sticks ). This light is also generated by chemiluminescence. The tubes contain three chemical components: an oxalic acid ester , a dye , the emission of which determines the color of the light, and a glass tube with hydrogen peroxide. If the tube is broken with hydrogen peroxide, the peroxyoxalate chemiluminescence starts .
The chemiluminescence of 1,2-dioxetanes is used in biochemistry and in medical diagnostics . They are able to reliably detect the smallest traces of enzymes down to individual molecules . In addition to other luminophores such as luminol , acridinium ester or luciferins , the concentrations of antibodies and antigens are determined in the chemiluminescence immunoassay . In addition, ATP and NADH can be determined in biological media down to very low concentrations. Sensitive detection systems have been developed for many substrates that are associated with ATP.
literature
- S. Albrecht, H. Brandl, Th. Zimmermann: Chemiluminescence . Hüthig Verlag, Heidelberg 1996
- Aldo Roda: Chemiluminescence and Bioluminescence . Royal Society of Chemistry 2011; ISBN 978-1-84755-812-1
Individual evidence
- ^ Duden : Spelling: chemiluminescence .
- ↑ Entry on chemiluminescence . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.C01045 Version: 2.3.1.
- ↑ Eilhard Wiedemann: About fluorescence and phosphorescence I. Treatise. In: Annals of Physics and Chemistry. 270, 1888, pp. 446-463, doi : 10.1002 / andp.18882700703 .
- ^ MM Rauhut, Chemiluminescence from Concerted Peroxide Decomposition Reactions, Acc. Chem. Research, 2, 80-87 (1969).
- ↑ AG Hadd, A.Seeber and JW Birks: Kinetics of Two Pathways in peroxyoxalates Chemiluminescence , Journal of Organic Chemistry, 65, (2000), from 2675 to 2683.
- ^ University of Jena : The peroxyoxlate chemiluminescence .
- ^ University of Jena: The chemiluminescence of 1,2-dioxetanes .
- ^ S. Albrecht, H. Brandl, W. Adam: chemiluminescence reactions, chemistry in our time, Volume 24, 1990, No. 5, pp. 227-238.
Web links
- Show experiments of luminol, oscillating chemiluminescence and oxalic acid esters (manufacture is also on the page)
- Chemical light - experiments on chemiluminescence Video recording of a lecture. From TIMMS, Tübingen Internet Multimedia Server of the University of Tübingen .