Oxalates
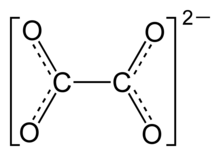
As oxalates , systematically Ethandioate , which are salts and esters of oxalic acid , respectively.
Salts
Many salty oxalates are sparingly soluble in water. Ammonium oxalate [(NH 4 ) 2 (COO) 2 ] and alkali oxalates such as sodium oxalate are most soluble in water . The precipitation of the poorly soluble calcium oxalate serves as a common detection of calcium ions. The anion of oxalic acid is also known as oxalate .
Since oxalic acid, but also its salts, form a sparingly soluble salt with calcium ( required for bone structure ), this can only be excreted slowly. Oxalic acid and its salts should therefore only be consumed in small doses . Oxalic acid is also found in chocolate, nuts, cocoa powder, spinach leaves, and rhubarb.
Analytics
Detection method for oxalates
The oxalate solution is buffered with acetic acid / acetate buffer , the pH range is between 4 and 6, then calcium chloride solution is added, the result is a white precipitate of calcium oxalate (in rhombic crystal form). Since a white precipitate is also formed by other ions, the precipitate is filtered off and dissolved in dilute sulfuric acid , a drop of potassium permanganate solution is added dropwise and the sample is heated. The solution, which is colored by potassium permanganate, must be discolored when heated.
Quantitative determination
In an aqueous solution, the concentration of oxalate ions can be determined by titration with KMnO 4 solution, but the oxalate solution must first be heated to 70 ° C. The following reaction occurs during the titration:
Ester
The esters of oxalic acid, also called oxalic acid esters , have the general formula R 1 O-CO-CO-OR 2 , where R are alkyl or aryl radicals. Acid esters with only one organic residue are not stable, but can exist as stable salts. The neutrally reacting, double esters are used as solvents. Important esters are dimethyl oxalate and diethyl oxalate .
Occurrence
Occurrence in plants
As a product of incomplete carbohydrate breakdown, oxalates are found in almost all plants.
Well-known plants with a very high proportion of oxalates are white goosefoot and the common sorrel . The roots and leaves of rhubarb and buckwheat also contain very high concentrations of oxalates.
Other edible plants with significant amounts of oxalate are the star fruit , black pepper , parsley , poppy seeds , amaranth , spinach , Swiss chard , beetroot , blueberries and most nuts. Also cocoa contains considerable amounts of oxalates. The leaves of the tea bush ( Camellia sinensis ) even take a top position in terms of oxalate content, although it must be taken into account that a tea made from these leaves ultimately only has comparatively low oxalate concentrations, on the one hand because of the small amount of tea leaves required for preparation are needed, on the other hand, because many oxalates are only sparingly soluble in water.
Occurrence in minerals
As salts of an organic acid, oxalates are only found in a few, rare minerals. The systematics of the minerals according to Strunz gives an overview. Even if the oxalates occurring as natural minerals (e.g. whewellite ) are salts of an organic acid, biological processes do not necessarily have to be involved in their formation - the possibility of the formation of organic substances (including amino acids) The purely abiotic path has now been confirmed by numerous experiments.
Physiological effects

In the body of higher organisms, the oxalate anions form small crystals with divalent metal ions such as calcium (Ca 2+ ) and divalent iron (Fe 2+ ) when excreted by the kidneys. Further aggregation can result in larger kidney stones . About 80% of all kidney stones consist of calcium oxalate . In addition to other kidney diseases, oxalates can also be the cause of gout , rheumatoid arthritis and vulvodynia .
Cadmium catalyzes the conversion of vitamin C into oxalic acid. This can happen in people who are exposed to high levels of cadmium, e.g. B. smokers, cause problems.
Individual evidence
- ^ Streitweiser, Andrew Jr .; Heathcock, Clayton H .: Introduction to Organic Chemistry , Macmillan 1976, p. 737.
- ↑ Coe FL, Evan A, Worcester E .: Kidney stone disease . In: J Clin Invest. . 115, No. 10, 2005, pp. 2598-608. doi : 10.1172 / JCI26662 . PMID 16200192 . PMC 1236703 (free full text).

