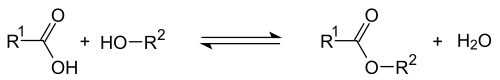
Carboxylic acid ester
| Carboxylic acid ester |
|---|

|
| R 1 and R 2 are organyl groups . However, R 1 can also be a hydrogen atom. The functional group is marked in blue . |
Carboxylic acid esters (R 1 -COO-R 2 ) are esters which are formally composed or formed from a carboxylic acid (R 1 -COOH) and an alcohol or a phenol (R 2 -OH). Esters of short-chain monocarboxylic acids with short-chain alcohols occur as fragrant compounds in essential oils (so-called fruit esters ) or, like ethyl acetate, they serve as a frequently used solvent. Esters of longer-chain monocarboxylic acids ( fatty acids with longer-chain alcohols) occur in natural waxes .
Vegetable and animal fats and oils are triple esters. They are called ( triglycerides ) because they are formed from the triple alcohol glycerin and three identical or different fatty acids.
The lactones are a special group of esters . These are cyclic esters that can form intramolecularly if the carboxy group of a carboxylic acid and the hydroxyl group of an alcohol are present in the same molecule and at the appropriate distance and can be reacted with one another. This is e.g. B. the case with the formation of γ-butyrolactone from γ-hydroxybutyric acid
The important plastics known as polyesters are polymeric carboxylic acid esters and can be formed from dicarboxylic acids and diols or from suitable lactones.
Nomenclature and structure explanation
Carboxylic acid esters are composed of a carboxylic acid part and an alcohol part . There are two possible types of common names for carboxylic acid esters. In the first type, the name of the ester is composed of the name of the carboxylic acid, the name for the organic residue of the alcohol, and the word ester as the name for the functional group . One example is the name ethyl acetate , which is formed from the name of the acid acetic acid , the name for the alkyl radical of the alcohol ethanol ( ethyl- ) and the word ester. This common name describes the structural makeup of the ester well. In the second type of trivial name, the carboxylic acid ester is understood as a salt of the carboxylic acid and is no longer referred to by name as an ester, but as a salt of the respective acid, in the case of acetic acid, as acetate . The ethanol ester of acetic acid is then referred to as ethyl acetate. This trivial name does not describe the structure of the ester, but it is short and haunting.
The systematic name of this ester, however, is ethyl ethanoate and is formed according to the principle “remainder of the alcohol (= ethyl ) + basic structure of the acid (= ethane ) + oat ”. However, the "R-oat" suffix is not allowed on all connections. If there is another functional group with higher priority in a compound, such as an acid or a cation , then the ester must be mentioned as a prefix . The designation to be used is R-oxycarbonyl- .
Examples
| acid | alcohol | Ester |
|---|---|---|
| simple carboxylic acid esters using the example of acetic acid | ||
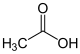 acetic acid |
Methanol |
 Methyl acetate Methyl acetate
|
|
Ethanol |
|
|
|
1-butanol |
|
|
| Lactones: internal carboxylic acid esters | ||
|
4-hydroxybutanoic acid |
|
|
| Fatty and fatty oils | ||
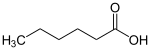 as an example: hexanoic acid (gen .: → fatty acids ) |
Glycerin |
 Triglycerides |
| Polyester: e.g. B. PET | ||
 Terephthalic acid |
Ethylene glycol |
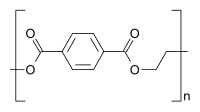 Polyethylene terephthalate |
Physical Properties
Due to the electronegativity difference between carbon and oxygen , the carboxylic acid ester group is polar and, in principle, enables solubility in water through the formation of hydrogen bonds . About 250 g / l of the short-chain carboxylic acid ester , methyl acetate, dissolve in water. Only about 10 g / l of the somewhat longer-chain butyl acetate go into solution. The total solubility is determined by the organic residues, so that if the residues are sufficiently non-polar, the water solubility is very low. Most esters are hydrophobic and are oils or waxes .
The boiling points of short-chain carboxylic acid esters are significantly lower compared to alcohols or carboxylic acids of comparable molar mass, since, unlike carboxylic acids or alcohols, they cannot form strong hydrogen bonds.
Reactions
synthesis
Alcohols and carboxylic acids can be converted to carboxylic acid esters by esterification . The esterification takes place using an acid as a catalyst and is an equilibrium reaction . By removing the resulting water from the reaction mixture, for. B. by azeotropic distillation or by using a molecular sieve , the equilibrium is shifted towards the ester.
In addition to the conversion of carboxylic acids, reactions of the corresponding carboxylic acid anhydrides and carboxylic acid chlorides also lead to esters. Another technically important reaction is transesterification , the exchange of one alcohol in the ester for another. This is used, for example, to produce the methyl esters of fatty acids from rapeseed oil , which are used as biodiesel .
A well-known reaction for the ester synthesis in organic chemistry is the Baeyer-Villinger oxidation . Here, percarboxylic acids are reacted with ketones to form carboxylic acid esters.
Ester cleavage
Esters can be split hydrolytically and pyrolytically. Bases , but also acids , are mostly used for the hydrolysis . When cleavage with acids, the alcohol and the carboxylic acid are formed, depending on the starting material used. If the ester is split with bases, the respective alcohol and the carboxylate anion are formed . Since the acidic hydrogen atom required for the reverse reaction is missing in the latter , this reaction is irreversible; H. irreversible. This makes it possible to use an excess of a starting material, e.g. B. alcohol, as a result of the principle of least compulsion according to Henry Le Chatelier to carry out a quantitative (i.e. complete) ester cleavage. The basic ester cleavage is called saponification .
The alkali salts of the fatty acids, i.e. the soaps , are obtained from fats and fatty oils by saponification . Ester cleavage can also take place with lipases , a group of enzymes that play an important role in the digestion of fats. The ester cleavage is of practical importance in detergents that contain lipases in order to break down the water-insoluble fat residues on textiles with the formation of fairly water-soluble fatty acids and readily water-soluble glycerol. To obtain enantiomerically pure carboxylic acids or alcohols, an enantiomer of racemic carboxylic acid esters is cleaved stereoselectively in chemistry . The second enantiomer of the carboxylic acid ester remains unchanged and allows racemate separation.
C, H acidity
Esters are not acids, but C, H-acidic compounds, as the proton in the α position can be split off (abstracted) by very strong bases . This creates a mesomeric-stabilized enolate :

(R 1 = H, organyl radical , such as, for example, an alkyl or aryl radical; R 2 = organyl radical, such as, for example, an alkyl or aryl radical).
Acid part versus alcohol part
As shown, in the (often common) formation of the ester from carboxylic acid and alcohol, the part of the ester shown in blue comes from the alcohol (here: ethanol ) and the part shown in red comes from the carboxylic acid (here: acetic acid ). An exception is the formation of esters from salts of carboxylic acids (carboxylates) and alkylating agents; the oxygen shown in blue in the picture comes from the carboxylic acid.
When esters are cleaved (both acidic and basic), the alcohol formed contains the oxygen shown in blue in the picture. There is also an exception here, namely the acid-catalyzed cleavage of esters of tertiary alcohols, in which an alkene is formed from the alcohol part ( E1 reaction ).
The fact that the oxygen atom remaining during ester formation comes from the alcohol can be proven by isotope labeling using the oxygen isotope 18 O.
More reactions
- Aryl esters can react to aryl ketones by means of the Fries rearrangement .
- Special esters can undergo a Chan rearrangement .
- Conversion into isocyanates by means of Lossen degradation .
Carboxylic acid ester occurrence in fruits (selection)
Pineapple contains ( S ) -2-methylbutyric acid ethyl ester
Apples contain 2-methylbutyrate, butyrate , acetic acid 2-methylbutyl ester , acetic acid n -butyl , and hexyl acetate .
Bananas contain amyl acetate , 3-methylbutyl butyrate, 3-methylbutyl 3-methylbutyrate
Strawberries contain anthranilic acid methyl ester , butyric acid ethyl ester, hexanoic acid ethyl ester
Passion fruit contains butyric acid ethyl ester, hexanoic acid ethyl ester, butyric acid hexyl ester , hexanoic acid hexyl ester
Peaches contain esters of acetic acid
Plums contain methyl cinnamate
use
Ethyl acetate is used as an extractant to decaffeinate coffee beans.
Individual evidence
- ↑ Baeyer-Villiger oxidation. Retrieved March 15, 2019 .
- ↑ Mary Adele J. O'Neil (ed.) The Merck Index. An Encyclopaedia of Chemicals, Drugs and Biologicals . 14th edition, Merck, Whitehouse Station 2006, ISBN 978-0-911910-00-1 , p. 955.
- ↑ Ivan Ernest: Binding, Structure and Reaction Mechanisms in Organic Chemistry. Springer, Vienna / New York 1972, ISBN 3-211-81060-9 , pp. 73-74.
- ↑ a b c H.-D. Belitz et al. a .: Textbook of food chemistry . 5th edition. Springer, Berlin a. a. 2001, pp. 821–825 ( limited preview in Google book search).
- ↑ a b c d Entry on fruit esters. In: Römpp Online . Georg Thieme Verlag, accessed on December 9, 2014.
- ↑ MJ Jordán u. a .: Aroma active components in aqueous kiwi fruit essence and kiwi fruit puree by GC-MS and multidimensional GC / GC-O . In: J. Agric. Food Chem. 2002, 50, 5386-5390. PMID 12207479 .
- ↑ W. Legrum: Fragrances, between stench and fragrance . Vieweg + Teubner, Wiesbaden 2011, p. 88 ( limited preview in the Google book search).
- ↑ R. Ebermann, I. Elmadfa: Textbook food chemistry and nutrition . 2nd Edition. Springer, Vienna and New York 2011, pp. 420–425 ( limited preview in Google book search).
- ↑ W. Ternes: Scientific basics of food preparation . 3. Edition. Behr's Verlag, Hamburg 2008, p. 824 ( limited preview in the Google book search).