Phenols
| Phenols |
|---|
|
phenol |
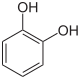 1,2-dihydroxybenzene ( catechol ) |
 1,3-dihydroxybenzene ( resorcinol ) |
|
1,4-dihydroxybenzene ( hydroquinone ) |
 1,2,3-trihydroxybenzene ( pyrogallol ) |
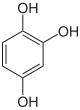 1,2,4-trihydroxybenzene ( hydroxyhydroquinone ) |
 1,3,5-trihydroxybenzene ( phloroglucinol ) |
 2,4,6-trinitrophenol ( picric acid ) |
In chemistry, phenols are compounds that consist of an aromatic ring ( arenes ) and one or more hydroxyl groups attached to it . According to chemical nomenclature , phenols are identified by adding the suffix -ol or preceding the prefix hydroxy- .
Phenols are produced by plants and microorganisms and also synthesized industrially .
properties
Due to the hydroxyl group, phenols are similar to alcohols , but react as weak acids in water and lead to slightly acidic solutions. The reason for the acidic character of the hydroxyl group is the stabilization of the phenolate anion formed by mesomerism . Phenols therefore form salts with bases , the phenolates . Despite the differences, phenols also undergo many reactions that are typical of alcohols, such as esterification with acids or the formation of phenol ethers .
Phenols have a keto-enol tautomerism , which, in contrast to the aliphatic ketones, is strongly on the enol side due to the energetic favored formation of the aromatic system (see Hückel rule ) .
Representative
Simple phenols
The phenol is the simplest of benzene derived phenol having only a hydroxy group and the molecular formula C 6 H 5 OH.
Other phenols having a hydroxyl group, so-called monovalent phenols are the toluene derivatives: the cresols ( o -, m - and p -cresol), and naphthols (α- and β-naphthol) and thymol .
The best-known dihydroxybenzenes (diphenols or dihydric phenols ), phenols with two hydroxyl groups, are catechol , resorcinol , hydroquinone and 1,4-naphthohydroquinone .
An important triphenol ( trivalent phenol ) is phloroglucin (1,3,5-trihydroxybenzene), which is used in hydrochloric acid solution as a detection reagent for the wood pulp lignin . Pyrogallol (1,2,3-trihydroxybenzene) is used in chemical analysis to detect and absorb oxygen . The third representative is hydroxyhydroquinone (1,2,4-trihydroxybenzene).
Substituted phenols
The picric acid is an example of a substituted phenol derivative. More important chemical phenol derivatives are: salicylic acid , o - and p -nitrophenol , gallic acid , eugenol , hexachlorophene , chloroxylenol , adrenaline and noradrenaline . The phenols also include tannins and many aromatic substances that determine the smell and taste of the wine . Other well-known phenols are, for example, vanillin , the most important aroma component of vanilla, and the vegetable pigments from the groups of flavonoids . The butylhydroxytoluene is a multi-applied anti-oxidant , which as a food additive is permitted E321.
Phenates
Phenolates are salts of metal cations and phenolate anions . The formula of the simplest phenolate is (M = metal ion; n corresponds to the valence of this metal ion). Phenolate ions are formed by deprotonation from phenols. Phenolate ions are weaker bases than alcoholate ions.
Occurrence and representation
Some phenols by distillation from the tar of stone and brown coal won, others can be isolated from natural products. Most phenols today - like the phenol itself - are synthesized using the cumene hydroperoxide process .
Research is currently being carried out into ways of producing phenols from renewable raw materials and especially from lignin . Various pyrolysis and hydrolysis processes are suitable for this purpose, for example .
use
Phenols are the basis for the production of synthetic resins ( phenoplasts ). They are also used in the manufacture of plastics , dyes , pharmaceuticals and pesticides . Hydroquinone (1,4-dihydroxybenzene) is a mild reducing agent and is therefore also used as a photographic developer .
proof
Most phenols are colored green-blue by iron (III) chloride . Specific detection is possible for many phenols by reacting them with benzoyl chloride to give corresponding, well-crystallizing esters .
Flavor carrier
Phenols also include tannins and other aromas that are important for the taste and smell of wine , for example . The tannins are mainly found in the skins and seeds of red grapes. A derivative of phenol, 2,4,6-trichloroanisole , is perceived as a cork tone in wines, with traces in the range of a few nanograms per liter being perceptible.
Phenols are also important flavor carriers in whiskey ; these include 4-ethylphenol , 4-ethylguaiacol , guaiacol , eugenol , syringaldehyde and vanillin , of which the first three are primarily responsible for the smoke note.
Phenolic yellowing
Phenolic yellowing refers to the yellowing of materials caused by nitrogen oxides and phenolic compounds.
Individual evidence
- ↑ Stephan Hättenschwiler, Peter M. Vitousek: The role of polyphenols in terrestrial ecosystem nutrient cycling . In: Trends in Ecology & Evolution . 15, No. 6, 2000, pp. 238-243. doi : 10.1016 / S0169-5347 (00) 01861-9 . PMID 10802549 .
- ↑ Paula Yurkanis Bruice: Organic Chemistry , 4th Edition, Pearson Education Inc., 2004, ISBN 0-13-121730-5 , S. 854th
- ^ Wissenschaft-Online-Lexika: Entry on phenols in the Lexikon der Chemie , accessed on February 17, 2009.
- ↑ a b Joseph Zakzeski, Pieter CA Bruijnincx, Anna L. Jongerius & Bert M. Weckhuysen: The Catalytic valorization of lignin for the Production of Renewable Chemicals . In: Chemical Reviews . tape 110 , no. 6 , 2010, p. 3565-3567 , doi : 10.1021 / cr900354u .
- ^ HP Latscha, HA Klein, G. Linti, GW Linti: Analytical Chemistry: Basic Chemistry III. , 4th edition, p. 127, Springer, 2004, ISBN 978-3-540-40291-6 .
- ↑ Entry on whiskey aroma. In: Römpp Online . Georg Thieme Verlag, accessed on June 15, 2014.
- ↑ DIN EN ISO 105-X18: 2007.
Web links
- Umweltlexikon-online.de: Phenols
- Phenols in virgin oil kill the stomach germ in the laboratory

