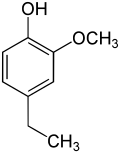
4-ethylguaiacol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 4-ethylguaiacol | ||||||||||||||||||
| other names | |||||||||||||||||||
| Molecular formula | C 9 H 12 O 2 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 152.19 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
15 ° C |
||||||||||||||||||
| boiling point |
234-236 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
4-Ethylguaiacol , also often abbreviated as 4-EG , is derived from guaiacol or 4-ethylphenol . This natural product is a phenolic compound with an attached ethyl group (–CH 2 –CH 3 ) and a methoxy group (–OCH 3 ). As a flavoring substance , it plays a special role in wine .
In addition to 4-ethylphenol, 4-ethylguaiacol is produced in wine by the yeast Brettanomyces bruxellensis or B. anomalus . These phenols are often found in red wines. 4-Ethylguaiacol is perceived with a spicy or clove aroma, its odor threshold in wine is at least 33 µg / l. In and of themselves, these aromas give the wine a good note. 4-Ethylguaiacol occurs together with 4-ethylphenol, which is perceived as having a paint odor, smelling of horse sweat, phenolic or leathery (the odor threshold is 440 µg / l). Hence, the mix of these phenols often contributes to a wine flaw . The relationship between the two components influences the (individual) sensory perception of the wine.
4-Ethylguaiacol is produced biochemically from ferulic acid in a two-step process . The latter is first decarboxylated to a vinyl intermediate and then oxidized to 4-ethylguaiacol . The first reaction is catalyzed by Brettanomyces by a hydroxycinnamic acid decarboxylase, the second by a vinylphenol reductase.
Individual evidence
- ↑ Entry on 4-ETHYLGUAIACOL in the CosIng database of the EU Commission, accessed on March 21, 2020.
- ↑ a b c d Data sheet 4-Ethylguaiacol from Sigma-Aldrich , accessed on March 18, 2011 ( PDF ).
- ^ Jamie Goode: The Science of Wine: From Vine to Glass . Univ. of California Pr 2006; ISBN 978-0-520-24800-7 ; P. 210.
- ↑ Kenneth C. Fugelsang and Charles G. Edwards: Wine Microbiology: Practical Applications and Procedures . Springer, Berlin; 2nd edition 2006. ISBN 978-0-387-33341-0 ; P. 164.
- ↑ Riccardo Flamini and Pietro Traldi: Mass Spectrometry in Grape and Wine Chemistry . John Wiley & Sons; 2010. ISBN 978-0-470-39247-8 ; P. 146.
- ↑ Riccardo Flamini and Pietro Traldi: Mass Spectrometry in Grape and Wine Chemistry . John Wiley & Sons; 2010. ISBN 978-0-470-39247-8 ; P. 145.
- ↑ Brettanomyces Monitoring by Analysis of 4-ethylphenol and 4-ethylguaiacol at etslabs.com
