Guaiacol
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Guaiacol | |||||||||||||||||||||
| other names | ||||||||||||||||||||||
| Molecular formula | C 7 H 8 O 2 | |||||||||||||||||||||
| Brief description |
colorless to yellowish oily liquid or solid with a characteristic odor |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 124.13 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
1.12 g cm −3 |
|||||||||||||||||||||
| Melting point |
27-29 ° C |
|||||||||||||||||||||
| boiling point |
205 ° C |
|||||||||||||||||||||
| Vapor pressure |
30 Pa (30 ° C); 13.73 Pa (20 ° C) |
|||||||||||||||||||||
| pK s value |
9.98 |
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| Refractive index |
1.5429 (20 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||||||||
Guaiacol is a secondary plant substance found in guaiac trees , which is structurally derived from anisole and phenol .
history
Guaiacol was in 1826 by Otto Unspoiled first by distillation of guaiac made.
Occurrence
Guaiacol is found in wood tar creosote and in wood tars , beech wood and guaiac resin have the greatest content . Along with other phenols, guaiacol is formed by thermal or microbial degradation of lignin or phenolic acids (e.g. ferulic acid ) and is therefore found in many foods, especially in smoked products. Guaiacol is one of the aromas in whiskey, and it can also be responsible for the cork tone in wine.
properties
Guaiacol has a smoky, medicinal smell and tastes sweet. The aroma threshold in water is 3 ppb . With its strong smoky note, guaiacol contributes significantly to the coffee aroma and the aroma of smoked foods. According to Antoine, the vapor pressure function results from ln (P) = A− (B / (T + C)) (P in kPa, T in K) with A = 13.6745, B = 3378.850 and C = −104.630 im Temperature range from 373 to 463 K. The enthalpy of vaporization at the boiling point is 52.7 kJ · mol −1 .
With iron (III) ions ( iron (III) chloride ) a green color can be determined.
The methoxy group has little influence on the acidity of the phenolic OH group , the pK s value has only a very small difference to the phenol (9.99) on.
presentation
Guaiacol 2 is by methylation of pyrocatechol (catechol) 1 , by means of dimethyl sulfate produced. The dimethylated product, veratrol, is also produced as a by- product :
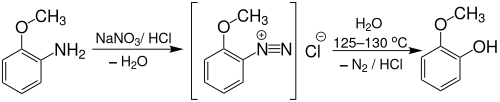
Gujacol can also be produced from o -anisidine by boiling (approx. 125 to 130 ° C) its diazonium salt :
use
Guaiacol is used in the fragrance industry to produce vanillin and eugenol . In the pharmaceutical industry, for example, it is used in drugs as an expectorant for bronchial diseases ( guaifenesin ). In coating technology it is used for the "anti-skinning" properties of inks and varnishes.
Web links
Individual evidence
- ↑ Entry on GUAIACOL in the CosIng database of the EU Commission, accessed on June 30, 2020.
- ↑ a b c d e f g h i Entry on guaiacol in the GESTIS substance database of the IFA , accessed on November 5, 2012(JavaScript required) .
- ^ Siegfried Hauptmann : Organic Chemistry , 2nd revised edition, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig, 1985, p. 340, ISBN 3-342-00280-8 .
- ↑ Zvi Rappoport (Ed.): CRC Handbook of Tables for Organic Compound Identification . 3. Edition. CRC Press , Boca Raton ( Florida ) 1984, ISBN 0-8493-0303-6 , Table 28: Acid Dissociation Constants of Phenols in Aqueous Solution (Listed in order of increasing pKa), p. 434 .
- ↑ Kazunori Miyamoto: Guaiacol. In: e-EROS Encyclopedia of Reagents for Organic Synthesis. May 27, 2014, John Wiley & Sons, Ltd. doi: 10.1002 / 047084289X.rn01705
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-332.
- ↑ Entry on Guaiacol in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Otto Unverdorben: About the guaiac resin . In: Annals of Physics and Chemistry . tape 92 , no. 6 , 1829, pp. 369-376 , doi : 10.1002 / andp.18290920620 .
- ↑ Horst Thielemann: Purity test of guaiacol (1-hydroxy-2-methoxybenzene) on simple and silver nitrate-impregnated sorption layers for thin-layer chromatography . In: Fresenius' Journal for Analytical Chemistry . tape 330 , no. 6 , 1988, pp. 530-530 , doi : 10.1007 / BF00490765 .
- ↑ a b entry on guaiacol. In: Römpp Online . Georg Thieme Verlag, accessed on November 11, 2014.
- ↑ George A. Burdock: Fenaroli's Handbook of Flavor Ingredients. Sixth Edition. CRC Press, Boca Raton 2010, ISBN 978-1-4200-9086-4 , pp. 774-775.
- ↑ Flavors whiskey on eyeforspirits.com, accessed on July 30, 2016.
- ^ A. Reynolds: Managing Wine Quality: Oenology and Wine Quality. Woodhead Publishing, 2010, ISBN 978-1-84569-998-7 , p. 393.
- ↑ Ming-Jer Lee, Chang-Ching Su, Ho-mu Lin: Vapor Pressures of Morpholine, Diethyl Methylmalonate, and Five Glycol Ethers at Temperatures up to 473.15 K. In: Journal of Chemical & Engineering Data . 50, 2005, pp. 1535-1538, doi: 10.1021 / je049627d .
- ↑ R. M. Stephenson, S. Malanowski: Handbook of the Thermodynamics of Organic Compounds. Springer 1987, ISBN 978-94-010-7923-5 , doi: 10.1007 / 978-94-009-3173-2 .
- ↑ CRC Handbook of Tables for Organic Compound Identification , Third Edition, 1984, ISBN 0-8493-0303-6 .
- ↑ a b Heinz Georg Osmar Becker, Werner Berger, Günter Domschke u. a .: Organikum . 19th edition. Johann Ambrosius Barth Verlag, LeipzigBerlinHeidelberg 1993, ISBN 3-335-00343-8 , p. 209, 564 .
- ↑ C.F. H. Allen and J. W. Gates, Jr .: o-Eugenol In: Organic Syntheses . 25, 1945, p. 49, doi : 10.15227 / orgsyn.025.0049 ; Coll. Vol. 3, 1955, p. 418 ( PDF ).
- ^ NIIR Board: The Complete Technology Book on Printing Inks. Asia Pacific Business Press Inc., 2003, ISBN 978-81-7833-048-8 , p. 490.