lignin
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|
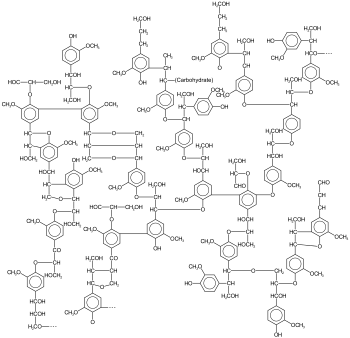 Example of a lignin structure |
|||||||
| General | |||||||
| Surname | lignin | ||||||
| CAS number | 9005-53-2 | ||||||
| Monomers / partial structures | Cumaryl alcohol , coniferyl alcohol , sinapyl alcohol | ||||||
| Type of polymer | |||||||
| Brief description |
off-white solid |
||||||
| properties | |||||||
| Physical state |
firmly |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Lignins ( lat. Lignum "wood") form a group of phenolic macromolecules , which are composed of different monomer components . There are solid biopolymers , which in the plant cell wall can be stored and thereby, the lignification of cell causing (lignification). About 20 to 30 percent of the dry matter of woody plants consists of lignins, which makes them the most common organic compounds on earth alongside cellulose and chitin . The total production of lignins is estimated at around 20 billion tons per year.
Lignins are essential for the compressive strength and durability of plant tissues, so the evolution of land-living plants and especially trees is very closely linked to the formation of lignin. Only with lignin can plants develop strengthening elements that ensure the stability of larger plant bodies outside the water. In the water, the relatively low density ensures static buoyancy .
function
As a support material and hardened polymer, lignin has a number of important functions for the plant. Lignins are essential for the strength of plant tissues, especially for their compressive strength, while the embedded cellulose fibers ensure tensile strength. Tear-resistant, flexible fibers (cellulose) are penetrated by a dense and rigid polymer as filler material (lignin). As analogies, technical materials such as reinforced concrete or natural fiber reinforced plastic are also structured accordingly.
Plants without lignin can withstand considerable tensile forces due to cellulose, but they are sensitive to pressure. Without lignin, no strengthening elements can be formed which, if there is no buoyancy caused by the water, guarantee the stability of larger plant bodies and build up corresponding supporting structures against the pressure caused by the force of weight . The formation of branches and branch systems to create large photosynthetically effective areas can only take place by stabilizing the branches.
Lignin also serves as a cement material for the cell network over the middle lamella . It offers protection against the penetration of water into the cell wall material and thus keeps it in the main vessels ( xylem and phloem ) as well as inside the cells. There is a further protective effect against UV light as well as mechanical damage and the penetration of pests. Finally, lignin can only be broken down with difficulty by bacteria or fungi and consequently inhibits the growth of pathogenic microorganisms passively and actively through the build-up of wound lignin in areas with mechanical damage. A similar structure with an analogous structure is represented by suberin , which occurs mainly in the cell walls of the phellem (cork).
The evolution of land-living plants and, above all, trees is closely linked to lignin biosynthesis: lignin is only found as real lignin when these plants appear, whereas in more primitive plants such as green algae only the building blocks or lignin-like polymers are present. The current assumption is that lignin is a new development and therefore a group-defining characteristic ( autapomorphy ) of vascular plants . It was probably first able to establish itself as a defense against fungal infections in the form of wound lignin and, based on this, assumed the central function as a stabilizing material. In 2009, however, lignin could also be detected in red algae of the species Calliarthron cheilosporioides . This raises the question of whether it originated convergently in both the higher plants and in the red algae, or whether it appeared early in the development of the eukaryotes and disappeared again in other organisms.
properties
Lignin is very firm to brittle and colored light to dark brown. It is optically isotropic , UV light is almost completely absorbed by the material, visible light is partially absorbed.
However, lignin is not a uniform substance, but a group of phenolic macromolecules made up of different monomer components . The combination of similar basic molecules creates a densely networked, amorphous mass. Compared to polysaccharides, the structure has significantly fewer polar groups , which means that lignins are hydrophobic and therefore not soluble in water and many other solvents. For this reason, they are more difficult to biodegrade than other natural substances, both chemically and biologically.
Structure and composition of lignin
Lignins are three-dimensional and amorphous networks (polymers) made up of aromatic basic building blocks that are linked to one another in a variety of ways. In addition to aromatic bonds, they contain many other carbon-carbon single and double bonds , and there are also many phenolic groups.
They are higher molecular weight ( relative molecular weight about 5000–10000) descendants of the phenylpropanoids , which as substituents of the benzene ring, in addition to a propane chain, are an OH or hydroxyl group , one or two OCH 3 or methoxy and various residual chains ( alkoxy or aryloxy groups ) contain. However, since the macromolecules grow in all spatial directions, with the central lamellae in particular allowing strong expansion and also being linked to one another secondarily, the lignin mass in a fully-grown tree probably corresponds to a single lignin polymer molecule, the mass of which is then several tons.
Depending on the type of wood , it is composed of structures that can be traced back to the basic building blocks p- coumaryl alcohol , coniferyl alcohol and sinapyl alcohol (monolignols) (see biosynthesis ). Since the lignin is created in a radical process in which the radical formation takes place enzymatically, but not their further reaction, the composition and the proportions of the individual building blocks are highly variable; there is no directional link that always follows the same pattern. In addition to the variability of each individual lignin molecule, the lignin of different wood and plant species also differs in the proportions of alcohols or the phenyl residues derived from them: Softwood lignin predominantly contains coniferyl units (around 90 percent), which is a guaiacyl residue (3-methoxy -4-hydroxyphenyl radical) and is therefore referred to as G-lignin. Hardwood lignin contains varying proportions of guaiacyl residues and sinapyl elements, which contain a syringyl residue (3,5-methoxy-4-hydroxyphenyl residue). The syringyl content can be between 5 and 65 percent, the resulting lignins are referred to as GS lignin. The lignin of the partially lignified grasses and other monocots is characterized by a high proportion of around 15 to 35 percent cumaryl elements, which form the para- hydroxy-phenylpropane and together with a syringyl proportion of the same amount and a guaiacyl proportion of 50 to 70 percent form the HGS lignins. In addition, cinnamic acids and cinnamaldehydes (the starting materials of the basic alcohols) are integrated into the matrix in small quantities.
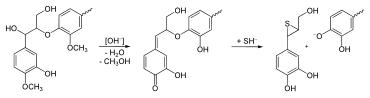
In the analysis , various detection reactions for lignin are known that are based on the structure of the fabric. To determine the presence of lignin, it turns red due to hydrochloric acid phloroglucinol solution . This reaction is due to the cinnamaldehydes embedded in the lignin matrix. Due to the different composition of the lignins in conifers and hardwoods, it is possible to differentiate between the two types of wood through the different coloration achieved with this verification. Softwoods turn cherry red, hardwoods red-violet. It can also be colored yellow with aniline / sulfuric acid and colored violet with Schiff's reagent . Gas chromatography is primarily used for qualitative analysis . The lignin content is determined using the Klason method, in which the polysaccharides are broken down by a two-stage acid hydrolysis and the remaining lignin residue is then weighed (Klasen lignin). In the case of GS lignins, UV spectroscopy of the acid solution is then necessary, as this contains acid-soluble components of the lignin.
Lignin content
| Hexoses (cellulose) |
Pentoses (hemicelluloses) |
Lignins | |
|---|---|---|---|
| Softwood | 57-60 | 7-11 | 27-32 |
| Birch wood | 45-47 | 21-27 | 19-20 |
| Beech wood | 50-54 | 19-24 | 22-23 |
| Wheat straw | 35-39 | 22-24 | 18-25 |
| Corn cobs | 37-44 | 32-35 | 15-19 |
| Bagasse | 42-50 | 29-42 | 16-21 |
The table on the right shows the proportions of lignin, cellulose and hemicelluloses in various biomass raw materials. These are primarily commercially relevant woods and lignocellulose-containing residues.
Lignin as cell wall reinforcement
Plant cell walls consist of cellulose fibrils that are bound into a matrix of pectins , hemicelluloses , proteins and lignin. The cellulose molecules, each made up of around 100 individual molecules, accumulate parallel to so-called elementary fibrils or micellar strands, which are stabilized by hydrogen bridges . 20 of these micellar strands together form a microfibril with a diameter of about 20 to 30 nanometers . The microfibrils, in turn, can combine to form macrofibrils with a diameter of around 400 nanometers, creating spaces of around 10 nanometers, which remain as interfibrillary spaces. The interfibrillary spaces serve, among other things, for the transport of water in the cell wall; in addition, larger molecules such as hemicelluloses, pectins and lignin are stored in these spaces to strengthen the cellulose structure ( incrustation ).
In most plant tissues, the lignin content is only about 1 percent, in plant parts lignified due to pressure loads it can amount to over 30 percent of the total mass; In these cases one speaks of lignocellulose . In addition to lignin, various mineral substances can also be responsible for incrustation, including silicates in grass, sedge and horsetail or calcium carbonate in calcareous algae .
In the case of lignification, the original cell wall matrix is replaced by the lignin polymer with the embedded cellulose fibers. The cellulose fibers are so tightly packed into the polymer that they can no longer move against each other and also lose their swellability . A special form of lignification takes place in so-called reaction wood: areas of wood that are exposed to particularly heavy loads are continuously strengthened. This reaction is different, however; In the case of horizontally growing branches of the conifers, for example, the pressure load leads to increased lignification of the underside of the branch from lignin-rich pressure wood . Deciduous trees, on the other hand, strengthen the top of the branch with cellulose-rich tensile wood without lignin content when subjected to the same stress.
Xylem lignification

Compressive stress occurs not only in construction elements, but also in the areas of the plant that have to withstand high internal pressure. This is especially the case in the channels for water transport in the trunk and in the roots, since here the water is transported against gravity and presses on the surrounding tissue. Correspondingly, lignification also forms here, which leads to cell wall tubes with a high proportion of lignin. The water-repellent (hydrophobic) character is an essential property of this function, as it prevents the water from escaping from the channels into the surrounding cell tissue and thus allows water to be transported over long distances.
These water-conducting elements of the xylem , which are differentiated into trachea and tracheid due to their size and structure , can be counted among the most important supporting structures in land plants together with the xylem-enhancing sclerenchyme .
biosynthesis
Biosynthesis of the lignin precursors
Lignin is a derivative of the phenylpropanoids, which in turn are derived from L - phenylalanine . By splitting off ammonia by a phenylalanine ammonia lyase (PAL) ( EC 4.3.1.5 ), the phenylalanine becomes cinnamic acid . This is converted to p- coumaryl- coenzyme A by further enzymes . This compound is the starting material for further modifications, for example hydroxylations on the aromatic ring and subsequent methylations . In the last step, the intermediate products bound to coenzyme A are reduced to the monolignols by a zinc-containing cinnam alcohol dehydrogenase (CAD) ( EC 1.1.1.195 ), whereby NADPH is always used as the reducing agent. These alcohols, which lead to the biosynthesis of lignin, are p- cumaryl alcohol (H unit), coniferyl alcohol (G unit) and sinapyl alcohol (S unit).
The composition of the lignins depends primarily on the proportion of the individual monolignols and the reaction conditions. In flowering plants , the lignin is composed in particular of sinapyl and coniferyl alcohol, in naked- seed plants coniferyl alcohol dominates, and grasses use all three monolignols. A key function is played by CAD, which due to its different substrate specificity is probably responsible for the different proportions of alcohols in the various plant groups: CAD from flowering plants and grasses reduces all three cinnamon aldehydes, while for CAD from naked plants sinapyl aldehyde is only a poor substrate and is accordingly less implemented.
Lignin synthesis
In 1948, Karl Freudenberg and his colleagues were able to produce an artificial lignin (dehydration polymer) from coniferyl alcohol and an extract from Agaricus campestris (field mushrooms ). A lignin that was later isolated from spruce showed chemical and physical properties similar to those of the artificial lignin. In this respect, it was indirectly proven that coniferyl alcohol is an essential component in the formation of spruce lignin. Further in-vivo investigations with radioactively labeled coniferyl alcohol or coniferin confirmed that these building blocks play an essential role in the biogenesis of lignin. A breakthrough in lignin research was achieved with these investigations.
Due to the composition of the individual building blocks and the diverse polymerisation options, lignins can have a wide variety of structures and accordingly form a whole class of compounds. They are only built up in the interfibrillary spaces from the alcohols that serve as precursors. How the monolignols are exported from the cell is not yet fully understood. These are probably transported to the outside as glucosides - glucocumaryl alcohol, coniferin and syringin . Here, the alcohols via their phenolic OH group glycosidically β- on sugar bound (glucose), and in this form are better soluble in water. In this way, the molecules can be transported through the plasmalemma and the apoplast of the cell and infiltrate into the cellulose spaces. Finally, the sugar molecules are split off by β- glycosidases from the cell wall. Such β-glycosidases have already been identified in some plants. Whether the monolignols diffuse passively through the cell wall or get out through a transport system is still the subject of research.
The exported monolignols are then spontaneously linked to an amorphous three-dimensional structure via an enzymatic oxidation-polymerization reaction. Here, the lignification begins at the corners and the central lamella of xylem cells. The polymerization process is catalyzed by extracellular peroxidases from hydrogen peroxide or laccases from oxygen , thereby forming phenoxy radicals. It is still unclear where the hydrogen peroxide comes from. The single electron is delocalized and stabilized over the entire molecule. This enables different points of connection for the formation of the reticulate lignin. Lignin contains chiral centers, but no optical activity can be detected using conventional methods.
Whether the networking can be controlled is still the subject of research. It is possible that extracellular glycoproteins, the conductor proteins , bring about a certain specificity in the crosslinking.
Lignin storage takes place in three phases. In the first phase, the macromolecule is deposited in the cell corners and the central lamella , after the pectin storage in the primary wall is complete. This is followed by progressive lignification of the S2 layer of the secondary cell wall. The main lignification takes place after the formation of the cellulose microfibrils in the S3 layer. The composition of the lignins varies within the three phases and thus also in the different layers.
Genetic modification of lignin biosynthesis
Since the removal of lignin from the wood for pulp production and especially for the production of biofuels ( cellulosic ethanol ) is one of the most complex production steps, there are various efforts to reduce the amount of lignin in the wood through green genetic engineering . This is mainly done by interfering with the genes necessary for the synthesis of the monomers , among other things by "switching off" the cinnam alcohol dehydrogenase (CAD) and the caffeic acid O-methyltransferase (COMT) by antisense RNA .
The corresponding techniques are currently still being researched on poplars and willows for cultivation in short rotation plantations and have not yet been implemented for technical implementation, but more effective delignification in the pulp process has already been proven. However, it has also been found that the effect of lignin reduction is not uniform, and that environmental factors are likely to have a greater impact on lignin production than genetic modification.
Lignin degradation
Lignin can be degraded both biologically and through various chemical-technical processes. When wood lignin biodegradation by bacteria and especially fungi (is decomposers ) decomposes . More highly organized living things are not able to break down lignin. Technical lignin digestion, on the other hand, is part of processes with the aim of separating lignin and cellulose in wood and utilizing them differently. Accordingly, it plays a major role in pulp production , wood saccharification and the use of lignocellulose in biorefineries . The common thermochemical methods of technical lignin degradation are very energy-intensive, pollute the environment and produce toxins.
If processed and uncoated wood is exposed to ultraviolet radiation over a long period of time , it is damaged on the surface, whereby the lignin in particular is denatured. In the case of direct weathering , it is subsequently washed out by rainwater. The surface then appears dirty-gray. If there is no rainwater, the wood takes on a silvery-white color as a result of the UV effect.
Biodegradation


Due to its complex network, lignin is a persistent natural substance and can only be decomposed very slowly by destructors. The formation of humus in the soil is largely promoted by the breakdown of lignin. Wood is broken down in two fractions, some of which run in parallel: Cellulose is broken down in the form of brown rot , in which the wood turns brown due to the lignin that remains, while lignin is broken down in the form of white rot , in which the wood changes color to light.
In the case of biological lignin degradation, a distinction is made between the utilization of already dissolved lignin fragments and the actual degradation of the natural substance. The former can already be used by many bacteria, especially actinomycetes and streptomycetes . White rot fungi such as the tinder fungus ( Fomes fomentarius ), the gray fire sponge ( Phellinus igniarius ), the butterfly tramete ( Trametes versicolor ) and Phanerochaete chrysosporium , however, enzymatically destroy the lignin content of the wood in order to utilize their actual substrate, cellulose or hemicelluloses. Correspondingly, with white rot, the wood turns white and becomes fibrous. Most of these mushrooms break down the lignin and the carbohydrates at the same time (simultaneous rot), the breakdown rates are also similarly high. Other fungi initially break down the lignin content more quickly, and cellulose accumulation occurs (successive white rot). This can be found, for example, in the mosaic layer fungus ( Xylobolus frustulatus ) or in the root sponge ( Heterobasidion annosum ), which causes red rot in spruce trees.
Lignin degradation always takes place under aerobic conditions and is very energy-intensive. Accordingly, it cannot serve as the sole source of carbon and energy. Therefore, white rot fungi is always a cometabolism in connection with other carbon sources. In order to break down, the fungi form thread-like hyphae that penetrate the lignin. Various enzymes are used to break down the lignin; they are exocytosed into the medium by the fungus and diffuse into the lignin. The degradation of the lignin is de facto a depolymerization and requires peroxidases and laccases, which act synergistically . In addition, oxygen, coenzymes, metals and complexing agents are required.
The fungi first release glyoxal , which is oxidized by a glyoxal oxidase to oxalic acid and hydrogen peroxide (H 2 O 2 ). H 2 O 2 is then reduced to water by a manganese peroxidase (MnP) ( EC 1.11.1.13 ), while manganese (II) (Mn 2+ ) is oxidized to Mn 3+ . Mn 3+ is chelated and easily penetrates the lignin as a small active oxidant. There, Mn 3+ can snatch individual electrons from the phenolic components of the lignin, so that a radical cation is formed. This is split into several fragments, often into benzaldehyde derivatives .
The radical cation can also be formed by a lignin peroxidase (LiP) ( EC 1.11.1.14 ). LiPs are heme- containing enzymes that can directly oxidize substituted aromatics, the main component in lignin. Not all white rot fungi code for LiPs, however. The peroxidase uses hydrogen peroxide as an oxidizing agent.
In the meantime, so-called hybrid enzymes have also been discovered in Pleurotus , Bjerkandera and other mushrooms, which are called “versatile peroxidases” (VP) ( EC 1.11.1.16 ). These have both manganese peroxidase and lignin peroxidase activity.
Finally, laccases ( EC 1.10.3.2 ) mainly oxidize low-molecular fragments of the lignin. They can generally enzymatically attack phenolic components of lignin. However, since these components only make up 10 percent of the lignin, the macromolecule is primarily used by the peroxidases mentioned above.
In addition, many other enzymes ( oxidoreductases , dehydrogenases ) are involved in lignin degradation .
Technical dismantling
Pulp production using the sulphate and sulphite process
Technical degradation of lignin plays an important role in pulp production. To produce cellulose, the lignin has to be dissolved and removed from the lignocellulose. There are different processes for cellulose digestion and for the subsequent pulp bleaching.
In around 80 percent of all pulp plants, digestion takes place via the so-called sulfate process , also known as the Kraft process. The lignin is broken down by hydrogen sulfide ions (HS - ) in a basic environment at around pH 13 through the use of sodium sulfide (Na 2 S) and sodium hydroxide (NaOH) or sodium hydroxide solution . The process takes about two hours at temperatures of about 170 ° C, but the ions also attack the cellulose and hemicelluloses , which means that only partial digestion is possible. The solid substance of the waste liquor from this process contains around 45 percent of the so-called Kraft lignin when using coniferous wood and around 38 percent when using hardwood.
An alternative is cellulose digestion using the sulphite process, in which the lignin is broken down by sulphonation . Ligninsulphonates , the salts of ligninsulphonic acid, are formed as a chemically not precisely defined reaction product of lignin with sulphurous acid . Calcium salts of lignin sulfonic acid are formed when the wood is broken down with calcium hydrogen sulfite solutions. Here, the solid substance of the waste liquor contains around 55 percent in the case of coniferous wood and around 42 percent in the form of lignosulfonate in the case of hardwood.
Lignin is also responsible for the yellowing of paper, which can therefore also be bleached with lignin-degrading enzymes such as laccase. However, bleaching is mainly carried out using chlorine bleaching or, nowadays, mostly "chlorine-free" with oxygen, chlorine dioxide , or less frequently hydrogen peroxide or ozone . In both cases, the residual lignin and the colorants present in the pulp are broken down by oxidation. This is particularly relevant for wood-containing paper, less so for wood-free paper . The terms wood-containing and wood-free are common in trade and colloquial language, but technically nonsensical, since paper made from the raw material wood always contains wood components. With wood-free paper these are just cellulose and hemicelluloses, with wood-containing paper the lignin-containing wood pulp . The terms lignin-containing and lignin-free would therefore be correct .
Wood saccharification
To transform wood into usable sugars (wood saccharification), various chemical, hydrothermal and enzymatic processes are used that remove the lignin from the wood and make the cellulose available.
Historically significant are mainly technical applications using acids , especially hydrochloric acid (HCl) or dilute sulfuric acid (H 2 SO 4 ), in which the shredded wood is cooked. Water molecules attach to the cellulose and form oligosaccharides , especially di- or trisaccharides , including glucose . Due to the hemicellulose and lignin present in the wood in addition to the cellulose, by-products or impurities are created which make the result almost exclusively usable for fermentation to alcohol or as a nutrient substrate for the fermentation of yeast . At times the wood brandy was produced in this way. For use in the chemical industry, the solution has to be cleaned and desalinated in a complex process.
Lignin solution for the biorefinery
In the context of the development of the biorefinery , saccharification is to take place using special enzymes, the cellulases , using a biotechnological method . It is hoped that the result will be fractions of cellulose that are as pure as possible for further saccharification, hemicelluloses and lignin so that all three components of the wood can be reused.
In order to get the individual fractions out of the wood as pure and undamaged as possible, special pretreatment is required. This takes place differently depending on the technical route and can, for example, be based on treatment with solvents such as ethanol (Organosolv process) or ionic liquids , the use of enzymes or steam treatment (Aquasolv process).
use

Apart from its use in the form of wood, lignin is mainly used as a by- product of the paper and pulp industry. Worldwide around 50 million tons of lignin are currently produced in this way every year. The resulting Kraft lignin and the lignin sulfonates are dissolved in the respective waste liquors and can be extracted from them. The main use for both types of lignin currently consists of energetic use, other uses are primarily for lignosulfonates from the sulfite process.
Basically, the various technical lignins differ in several properties that can influence their use. The main difference lies in the molecular size: Kraft lignin has a molar mass of 2000 to 3000 g / mol, while lignosulfonates have a molar mass of 20,000 to 50,000 g / mol. Organosolv lignin is 1000 to 2000 g / mol. Lignosulfonates also contain a sulfur content of 4 to 8 percent and a few phenolic hydroxyl groups (–OH) compared to 1 to 1.5 percent sulfur and many phenolic hydroxyl groups in Kraft lignin and many phenolic hydroxide ions (OH - ) without sulfur in Organosolv lignin.
The properties of lignin modified by oxidative ammonolysis as a humus substitute are intensively investigated. The nitrogenous lignins are similar in structure to humic substances and are suitable as depot fertilizers. The N-Lignins are also suitable for the recultivation of post-mining landscapes .
The direct use of technical lignins as raw products is very limited, as there are a number of disadvantages that oppose this. Due to its very complex structure and the associated inhomogeneity, lignin can only be used to a very limited extent for applications, as more precisely defined properties of the raw material are usually required. In addition, there is the high degree of contamination in the waste liquors and the high sulfur content in the lignin types, which make complex cleaning steps necessary. The resulting very complex extraction from the waste liquor has led to the fact that unpurified technical lignin has essentially only been used for lower-value applications such as energy use or as a non-specific adhesive component and as a dispersant . Further material uses are either in the direct use of lignosulfonates or in chemical modification through the use of pyrolysis , hydrolysis or hydrogenolysis for the production of various chemicals. These paths are also time-consuming and are therefore rarely used.
Lignin in energetic use
The lignin, which occurs in large quantities as a residue in paper production, especially in the sulphate process, is used as black liquor , primarily as a fuel directly in the pulp mills. It has a calorific value of 23.4 MJ / kg and, in addition to generating energy for the factories themselves, covers 80 to 100 percent of their energy requirements, it also serves to optimize profits through the sale of heat and electricity.
In the production of wood pellets as an energy source, the wood's own lignin forms the binding agent. Finely ground wood is heated during pressing, the lignin liquefies and binds the wood particles together when it cools. Fresh pellets therefore still smell strongly of lignin.
Use of lignosulfonates
Large quantities of lignosulfonates are used in a wide range of applications, which primarily take advantage of their properties as a polyelectrolyte , their adsorption properties , their low viscosity and their dark color. They are physiological and relatively harmless to the environment, which means that they are also used in sensitive areas. The main part of the production of around 1,000,000 tons per year ( tpd ) is used as a dispersant in concrete and cement (around 100,000 tpd), as an additive to drilling fluids (around 100,000 tpd) and as a binding agent in pellets for animal feed , in fertilizers and other agrochemicals , chipboard , briquettes as well as in printing ink and foundry sand cores . Lignosulfonates are also used as a paper additive, as a dispersant and emulsifier in paints and varnishes and as an additive in plaster of paris and tanning agents.
Recent developments in lignosulfonate chemistry use the polyelectrolytic properties of lignin and are aimed at use in medicine, fine chemistry and the improvement of soil water storage.
Lignin as a biomaterial
As a natural substance, lignin is a highly complex macromolecule (polymer), and this structure can be used as a biomaterial . The kraft lignin from the sulphate process in paper production must first be cleaned, which is why there are still few approaches to manufacture lignin-based polymers.
In 1998 the company Tecnaro developed a natural biomaterial that was given the name Arboform and is commonly referred to as "liquid wood". It is based on lignin, to which natural fibers such as flax or hemp are added, and can be processed with established plastics processing forms, in particular in injection molding , extrusion , pressing processes as well as deep drawing and blow molding .
Both lignin and various lignin derivatives can be used as building blocks in thermosets or as fillers in plastics . They act as a phenolic resin component . By reacting with epichlorohydrin , epoxy resins can be produced which, when condensed with alkali lignin, result in polyalcohols . With isocyanates , these can be converted into polyurethanes . When lignin reacts with formaldehyde , phenoplasts are formed , and when crosslinking with copolymers such as urea , melamine and furans via formaldehyde, various resins are produced (urea-formaldehyde resins, melamine resins, and furan resins or syntactics). Lignin-based phenoplasts in particular represent a potential alternative to phenols and formaldehyde, which are harmful to health, as binders in chipboard and other wood-based materials ; Due to their high molecular structure, they are less volatile and soluble, and they are also classified as physiologically harmless.
Lignin in the chemical industry and biorefinery
Although lignin does not play a major role in the production of chemicals today, the raw material is predicted to have great potential in the future. In the last few years in particular, research has focused on the use of lignin in the pulp industry and in the (still hypothetical) biorefinery . The aim of the research is to obtain the highest possible quality products from the lignin.
Lignin is already being used to produce vanillin , which is used as a nature-identical flavoring substance for vanilla . It is produced during the oxidation of lignin sulfonates , which in turn is obtained from lignin through acid hydrolysis. Various phenols , carboxylic acids , tar and dimethyl sulfide (DMS) can be produced from lignin by means of an alkali melt . DMS can also be produced via alkaline demethylation and can be further oxidized to dimethyl sulfoxide (DMSO), an important solvent. In turn, hydrogenolysis can also produce phenols, tar, benzene and oils.
Another important option for the future use of lignin is pyrolysis , a process for the thermal breakdown of organic compounds at high temperatures. It is possible by pyrolysis at temperatures of 400 to 500 ° C phenols, methane , carbon monoxide and carbon gain. At temperatures of 700 to 1,000 ° C, lignin can be split into syngas , ethene and benzene , and acetylene is produced in an arc pyrolysis .
Energy storage
The chemical properties of lignin in combination with its environmental friendliness, its wide availability and its low costs make lignin a very promising raw material for metal-free redox flow batteries for energy storage , especially for the stationary storage of electricity from renewable energies .
Evidence cited
- ↑ Entry on lignin. In: Römpp Online . Georg Thieme Verlag, accessed on June 14, 2012.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b c d e lignin. In: Sitte et al. 2002, pp. 353-356.
- ↑ After Lüttge / Kluge / Bauer 1994, p 217th
- ↑ a b c d e f Secondary walls of fiber and wood cells. In: Sitte et al. 2002, pp. 95-96.
- ^ A b c Peter Raven, Ray F. Evert, Susan Eichhorn: Biology of plants . 4th edition. de Gruyter , Berlin / New York 2006, ISBN 3-11-018531-8 , pp. 38 (English: Biology of Plants . Translated by B. Biskup et al.).
- ↑ a b c d Hans W. Heldt and Birgit Piechulla: Plant biochemistry . 4th edition. Spektrum Akademischer Verlag, 2008, ISBN 978-3-8274-1961-3 , p. 420-422 .
- ↑ CF Delwiche, LE Graham, N. Thomson: Lignin-like compounds and sporopollenin in Coleochaete, an algal model for land plant ancestry. Science 245: 399-401 (1989).
- ↑ Peter Raven, Ray F. Evert, Susan Eichhorn: Biology of plants . 4th edition. de Gruyter , Berlin / New York 2006, ISBN 3-11-018531-8 , pp. 398 (English: Biology of Plants . Translated by B. Biskup et al.).
- ↑ Patrick T. Martone1, José M. Estevez, Fachuang Lu, Katia Ruel, Mark W. Denny, Chris Somerville, John Ralph: Discovery of Lignin in Seaweed Reveals Convergent Evolution of Cell-Wall Architecture. Current Biology 19 (2), Jan. 27, 2009, pp. 169-175.
- ↑ a b c Oskar Faix: Chemistry of wood. In: André Wagenführ, Frieder Scholz (Hrsg.): Taschenbuch der Holztechnik. Fachbuchverlag at Carl Hanser Verlag, Leipzig 2008, ISBN 978-3-446-22852-8 , pp. 127-259.
- ↑ a b The lignified plant cell. In: Schopfer & Brennicke 1999, p. 33.
- ^ Hans Günther Hirschberg: Handbook of process engineering and plant construction. Springer Verlag 1999, p. 436.
- ↑ Jaakko Pöyry Consulting Oy: Non-wood fiber. Helsinki 1998.
- ↑ a b According to Lüttge / Kluge / Bauer 1994, p. 153.
- ↑ a b Marjamaa, K. et al . (2009): The role of xylem class III peroxidases in lignification . In: J Exp Bot 60 (2), pp. 367-376; PMID 19264758 ; doi: 10.1093 / jxb / ern278 .
- ↑ a b Gerhard Michal (Ed.): Biochemical pathways. Biochemistry Atlas . Spektrum Akademischer Verlag, Heidelberg 1999, ISBN 3-86025-239-9 , p. 64 .
- ^ A b Peter Karlson , Detlef Doenecke , Jan Koolman , Georg Fuchs , Wolfgang Gerok : Karlsons Biochemie und Pathobiochemie . 15th revised u. redesigned edition. Thieme, Stuttgart 2005, ISBN 3-13-357815-4 , p. 451 .
- ↑ Ralph, J. et al . (1999): Are lignins optically active? In: J Agric Food Chem 47 (8), pp. 2991-2996; PMID 10552598 ; doi: 10.1021 / jf9901136 .
- ↑ Davin, LB. et al. (1997): Stereoselective bimolecular phenoxy radical coupling by an auxiliary (conductor) protein without an active center . In: Science 275 (5298), pp. 362-366; PMID 8994027 ; doi: 10.1126 / science.275.5298.362 .
- ↑ Laurence B. Davin, Norman G. Lewis: Conductor Proteins and Conductor Sites Explain the Mystery of Specificity of Radical Precursor Coupling in Lignan and Lignin Biosynthesis. Plant Physiology 123, 2000, pp. 453-461.
- ↑ Ronald Hatfield, Wilfred Vermerris: Lignin Formation in Plants. The Dilemma of Linkage Specificity. Plant Physiology 126, 2001, pp. 1351-1357.
- ↑ Ronald J. Dinus: Genetic improvement of poplar feedstock quality for ethanol production. Applied Biochemistry and Biotechnology, 2001, pp. 23-34; doi: 10.1385 / ABAB: 91-93: 1-9: 23 .
- ^ A b G. Pilate, E. Guiney, K. Holt, M. Petit-Conil, C. Lapierre, JC Leplé, B. Pollet, I. Mila, EA Webster, HG Marstorp, DW Hopkins, L. Jouanin, W Boerjan, W. Schuch, D. Cornu, C. Halpin: Field and pulping performances of transgenic trees with altered lignification. Nature Biotechnology , June 20, 2002, pp. 607-612. PMID 12042866 .
- ↑ EL Tilstona, C. Halpin, DW Hopkin: Genetic modifications to lignin biosynthesis in field-grown poplar trees have inconsistent effects on the rate of woody trunk decomposition. Soil Biology and Biochemistry 36 (11), Nov. 2004, pp. 1903-1906; doi: 10.1016 / j.soilbio.2004.05.010 .
- ^ A b Hans G. Schlegel: General microbiology . Ed .: Georg Fuchs. 8th edition. Thieme, Stuttgart 2007, ISBN 3-13-444608-1 , p. 293-294 .
- ↑ Singh, D. and Chen S. (2008): The white-rot fungus Phanerochaete chrysosporium: conditions for the production of lignin-degrading enzymes . In: Appl Microbiol Biotechnol 81 (3), pp. 399-417; PMID 18810426 ; doi: 10.1007 / s00253-008-1706-9 .
- ^ Olaf Schmidt: Wood and tree mushrooms. Biology, damage, protection, benefit . Springer, Heidelberg, Berlin 1994, ISBN 3-540-57334-8 .
- ↑ a b c Katharina Munk (ed.): Pocket textbook Biology: Microbiology . Thieme, Stuttgart 2008, ISBN 978-3-13-144861-3 , p. 507-508 .
- ↑ David N.-S. Hon, Nobuo Shiraishi: Wood and cellulosic chemistry ( limited preview in Google book search).
- ↑ a b c Mutton, KE. and Cullen, D. (2008): Role of fungal peroxidases in biological ligninolysis . In: Curr Opin Plant Biol 11 (3), pp. 349-355; PMID 18359268 ; doi: 10.1016 / j.pbi.2008.02.003 .
- ↑ a b Martínez, AT. et al . (2005): Biodegradation of lignocellulosics: microbial, chemical, and enzymatic aspects of the fungal attack of lignin . In: Int Microbiol 8 (3), pp. 195-204; PMID 16200498 ; PDF (full text, English)
- ↑ a b c d e Birgit Kamm, Michael Kamm, Matthias Schmidt, Thomas Hirth, Margit Schulze: Lignocellulose-based Chemical Products and Product Family Trees . In: Birgit Kamm, Patrick R. Gruber, Michael Kamm (eds.): Biorefineries - industrial processes and products. Status quo and future directions . Wiley-VCH, Weinheim 2006, ISBN 3-527-31027-4 , pp. 67-84 .
- ↑ a b c d Erich Gruber: Macromolecular chemistry, ecology and economy of renewable raw materials: Use of lignin. ( Memento of July 27, 2004 in the Internet Archive ) Documents for the lecture winter semester 1999/2000.
- ↑ University of Natural Resources and Life Sciences, Vienna research portal . research.boku.ac.at. Retrieved May 7, 2009.
- ↑ Investigations into the suitability of new humus substitutes as soil improvers in the recultivation of mining areas and the remediation of problem areas - joint BMBF research project - . fib-finsterwalde.de. Retrieved May 7, 2009.
- ↑ Modified lignosulfonic acids (LSS) as binders for the production of weather-resistant wood-based materials (PDF; 2.4 MB), Ralph Lehnen and Okko Ringena, work report of the Institute for Wood Chemistry and Chemical Technology of Wood.
- ↑ company website Tecnaro
- ↑ nova-Institut GmbH, University of Bremen (ed.): BIB'09 - Industry Leader Innovative Biomaterials 2009. Hürth 2009, pp. 62–63.
- ^ JJ Bozell, JE Holladay, D. Johnson, JF White: Top Value Added Chemicals From Biomass. Volume II — Results of Screening for Potential Candidates from Biorefinery Lignin. Pacific Northwest National Laboratory (PNNL) and the National Renewable Energy Laboratory (NREL), October 2007 ( PDF ).
- ↑ Transparent and heat-stable polyamide - 100 percent bio-based. In: fraunhofer.de , August 29, 2018, accessed on July 31, 2020.
- ↑ Alolika Mukhopadhyay et al .: Metal-Free Aqueous Flow Battery with Novel Ultrafiltered lignin as Electrolyte . In: ACS Sustainable Chemistry & Engineering . tape 6 , no. 4 , 2018, p. 5394-5400 , doi : 10.1021 / acssuschemeng.8b00221 .
literature
- Peter Sitte , Elmar Weiler , Joachim W. Kadereit , Andreas Bresinsky , Christian Körner : Textbook of botany for universities . Founded by Eduard Strasburger . 35th edition. Spektrum Akademischer Verlag, Heidelberg 2002, ISBN 3-8274-1010-X .
- Ulrich Lüttge, Manfred Kluge, Gabriela Bauer: Botany . 2nd Edition. VCH, Weinheim u. a. 1994, ISBN 3-527-30031-7 .
- Peter Schopfer, Axel Brennicke: Plant Physiology . 4th edition. Springer, Heidelberg, Berlin 1999, ISBN 3-540-64231-5 .
- Lincoln Taiz, Eduardo Zeiger: Plant physiology. The original with translation aids . 4th edition. Spectrum Academic Publishing House, Heidelberg, Berlin 2007, ISBN 978-3-8274-1865-4 .
- Peter Nuhn: Natural Products Chemistry. Microbial, vegetable and animal natural substances . with the assistance of Ludger Wessjohann. 4th edition. S. Hirzel Verlag, Stuttgart 2006, ISBN 3-7776-1363-0 , p. 320-322 .
- W. Boerjan, J. Ralph, M. Baucher (2003): Lignin biosynthesis . In: Annu Rev Plant Biol 54, pp. 519-546; PMID 14503002 ; doi: 10.1146 / annurev.arplant.54.031902.134938
- Gerhard Krüger: Lignin - its meaning and biogenesis, chemistry in our time , 10th year 1976, No. 1, pp. 21-29, doi: 10.1002 / ciuz.19760100104