Dimethyl sulfoxide
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Dimethyl sulfoxide | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 2 H 6 OS | |||||||||||||||||||||
| Brief description |
colorless and odorless liquid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 78.13 g mol −1 | |||||||||||||||||||||
| Physical state |
liquid |
|||||||||||||||||||||
| density |
1.10 g cm −3 (20 ° C) |
|||||||||||||||||||||
| Melting point |
18 ° C |
|||||||||||||||||||||
| boiling point |
189 ° C |
|||||||||||||||||||||
| Vapor pressure |
|
|||||||||||||||||||||
| solubility |
miscible with water, alcohols , acetone , chloroform and benzene , but not with alkanes |
|||||||||||||||||||||
| Dipole moment | ||||||||||||||||||||||
| Refractive index |
1.4793 (20 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| MAK |
Switzerland: 50 ml m −3 or 160 mg m −3 |
|||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| Thermodynamic properties | ||||||||||||||||||||||
| ΔH f 0 |
−204.2 kJ / mol |
|||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||||||||
Dimethyl sulfoxide (abbreviation DMSO ) is an organic solvent and belongs to the class of sulfoxides .
Presentation and extraction
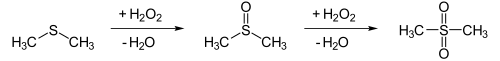
Technically, dimethyl sulfoxide is made from dimethyl sulfide z. B. produced by catalytic oxidation with dinitrogen tetroxide in the presence of oxygen . In the laboratory, the thioether dimethyl sulfide can be oxidized with stoichiometric amounts of hydrogen peroxide or dilute nitric acid. The dimethyl sulfoxide can, however, be further oxidized to dimethyl sulfone :
DMSO is also a by-product in pulp production .
properties
Physical Properties
Dimethyl sulfoxide is a colorless, odorless, hygroscopic liquid. After prolonged storage, it often has a foul odor (of dimethyl sulfide ). With a melting point of 18 ° C, the substance can only solidify a little below room temperature. The enthalpy of fusion is 14.37 kJ mol −1 . The compound boils at 189 ° C. under normal pressure. According to Antoine, the vapor pressure function results from log 10 (P) = A− (B / (T + C)) (P in bar, T in K) with A = 4.49107, B = 1807.002 and C = −60.995 in the temperature range from 325.5 to 442.1 K. or with A = 5.23039, B = 2239.161 and C = −29.215 in the temperature range from 293 to 323 K. It can be mixed with water in any ratio , and with many organic ones Solvents such as alcohols , carboxylic acid esters , ketones and chlorinated hydrocarbons . It belongs to the group of nucleophilic , aprotic , dipolar solvents (such as dimethylformamide ).
Chemical properties
The compound begins to thermally decompose at the normal pressure boiling point at 189 ° C, which can be violent or explosive. The decomposition is catalyzed by acids or bases, so that it can become relevant even at significantly lower temperatures. Violent to explosive decomposition occurs in the presence of halogen compounds, metal nitrates , metal perchlorates , sodium hydride , periodic acid and fluorinating agents.
Dimethyl sulfoxide can be deprotonated with sodium hydride or sodium amide to form a methyl sulfinyl carbanion (dimsyl anion), which is used as a very strong nucleophilic reagent in organic synthesis. The reaction mixtures can decompose explosively above 70 ° C. There is also a risk of explosion when isolating the solid sodium salt.
Safety-related parameters
Dimethyl sulfoxide forms flammable vapor-air mixtures above the flash point at 88 ° C. The lower explosion limit is 1.8 vol.% (58 g / m³). An upper explosion limit (UEL) cannot be determined due to the thermal decomposition of the substance. The limiting oxygen concentration was determined to be 3.9% by volume at 200 ° C. The ignition temperature is 270 ° C. The substance therefore falls into temperature class T3. The electrical conductivity of 2 · 10 −7 S · m −1 is rather low.
DMSO-d 6
Completely deuterated DMSO ( DMSO- d 6 ) - in which all six hydrogen atoms have been replaced by deuterium - is used as a solvent in NMR spectroscopy .
pharmacology
Dimethyl sulfoxide has anti-inflammatory ( anti-inflammatory ) and pain ( analgesic ) properties. It is therefore used therapeutically as a percutaneous (Latin: through the skin ) drug for the treatment of local pain conditions (for example in the case of sports injuries or rheumatic complaints). Since DMSO contributes to the rapid swelling of bruises , it is especially used in martial arts when necessary. A study from Brazil was able to show that DMSO gel and therapeutic ultrasound have significantly better results in terms of swelling of blunt injuries compared to other forms of treatment (or no treatment).
Its special ability is easy penetration into skin and other cell membranes. It is therefore used as a carrier substance for drugs applied to the skin ( ointments , gels , plasters , tinctures ) to infiltrate active ingredients such as heparin or analgesics as a so-called transport mediator (also called penetration enhancers, carrier substances ), i.e. That is, substances dissolved in DMSO are easily absorbed by the organism through the skin. This also applies to poisons that are otherwise no or weakly effective contact poisons, such as cyanides . For this reason, solutions of compounds considered to be toxic must be rinsed off immediately with suitable agents (e.g. water) if they come into contact with the skin.
use
DMSO is a widely used solvent in laboratories and technology. It is used in spinning solutions of polyacrylonitrile , as a paint remover , as a solvent in aromatic extraction and as a reaction medium in organic syntheses. Many inorganic salts also have good solubility in DMSO.
In synthetic organic chemistry, it is used as an oxidizing agent in the Swern oxidation and the Parikh-Doering oxidation. In pharmacy it is used as a component of ointments (see properties).
In cell culture , DMSO is used in freezing media for the cryopreservation of eukaryotic cells . As an anti-freeze agent , it prevents the formation of ice crystals during the freezing process ; these can destroy cell organelles and thus lead to cell death. DMSO inhibits crystal formation a little better than glycerine .
Most substance libraries also use DMSO as a solvent. However, since the solvent is not completely inert , this can lead to a deterioration in sample purity.
Individual evidence
- ↑ a b c d e f g h Entry for CAS no. 67-68-5 in the GESTIS substance database of the IFA , accessed on April 17, 2018(JavaScript required) .
- ↑ Hans Beyer, Wolfgang Walter: Textbook of organic chemistry. 23rd edition. S. Hirzel Verlag, Stuttgart 1998, ISBN 3-7776-0808-4 .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Dipole Moments, pp. 9-55.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-210.
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 67-68-5 or dimethyl sulfoxide ), accessed on November 2, 2015.
- ↑ Entry on dimethyl sulfoxide in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on July 31, 2018 or earlier.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-22.
- ↑ a b c d e Entry on dimethyl sulfoxide. In: Römpp Online . Georg Thieme Verlag, accessed on June 14, 2014.
- ↑ TB Douglas: Heats of Formation of Liquid Methyl Sulfoxide and Crystalline Methyl Sulfone at 18 deg. In: J. Am. Chem. Soc. 68, 1946, pp. 1072-1076, doi: 10.1021 / ja01210a046 .
- ↑ HL Clever, EF Westrum, Jr .: Dimethylsulfoxide and dimethylsulfone. Heat capacities, enthalpies of fusion, and thermodynamic properties. In: J. Phys. Chem. 74, 1970, pp. 1309-1317, doi: 10.1021 / j100701a027 .
- ↑ B. Kaczmarek, A. Radecki: Vapor-Liquid Equilibria in Binary Systems Containing Ethanol with Tetramethyldisiloxane and Dimethyl Sulfoxide. In: J. Chem. Eng. Data . 34, 1989, pp. 195-197, doi: 10.1021 / je00056a014 .
- ↑ G. Jakli, WA van Hook: The Vapor Pressures of Dimethyl Sulfoxide and Hexadeuterodimethyl Sulfoxide from about 313 to 453 K. In: J. Chem. Thermodyn. 4, 1972, pp. 857-864, doi: 10.1016 / 0021-9614 (72) 90007-9 .
- ^ TB Douglas: Vapor Pressure of Methyl Sulfoxide from 20 to 50 °. Calculation of the Heat of Vaporization. In: J. Am. Chem. Soc. 70, 1948, pp. 2001-2002, doi: 10.1021 / ja01186a005 .
- ↑ a b c d Roth / Weller: Dangerous chemical reactions. ecomed Sicherheit, Hüthig Jehle Rehm Publishing Group, Landsberg / Lech, 31. Supplementary delivery 8/2000.
- ↑ I. Iwai, J. Ide: 2,3-Diphenyl-1,3-Butadiene In: Organic Syntheses . 50, 1970, p. 62, doi : 10.15227 / orgsyn.050.0062 ; Coll. Vol. 6, 1988, p. 531 ( PDF ).
- ↑ EM Kaiser, RD Beard, CR Hauser: Preparation and reactions of the mono- and dialkali salts of dimethyl sulfone, dimethyl sulfoxide, and related compounds. In: J. Organomet. Chem. 59, 1973, p. 53. doi: 10.1016 / S0022-328X (00) 95020-4 .
- ↑ a b c E. Brandes, W. Möller: Safety-related parameters. Volume 1: Flammable Liquids and Gases. Wirtschaftsverlag NW - Verlag für neue Wissenschaft, Bremerhaven 2003, ISBN 3-89701-745-8 .
- ↑ Osterberg PM, JK Niemeier, CJ Welch, JM Hawkins, JR Martinelli, TE Johnson, TW Root, SS Stahl: Experimental Limiting Oxygen Concentrations for Nine Organic Solvents at Temperatures and Pressures Relevant to Aerobic Oxidations in the Pharmaceutical Industry. In: Org. Process Res. Dev. 19, 2015, pp. 1537–1542. doi: 10.1021 / op500328f
- ↑ Technical rule for hazardous substances TRGS 727, BG RCI leaflet T033 Avoidance of ignition hazards due to electrostatic charges , status August 2016, Jedermann-Verlag Heidelberg, ISBN 978-3-86825-103-6 .
- ^ Arnd Krüger : DMSO. In: competitive sport. 43, 3, 2013, p. 28.
- ↑ Paulo CL Silveira, Eduardo G. Victor, Débora Schefer, Luciano A. Silva, Emilio L. Streck, Marcos M. Paula, Ricardo A. Pinho: Effects of therapeutic pulsed ultrasound and dimethylsulfoxide (DMSO) phonophoresis on parameters of oxidative stress in traumatized muscle . In: Ultrasound in Medicine & Biology . tape 36 , no. 1 , 2010, p. 44-50 , doi : 10.1016 / j.ultrasmedbio.2009.09.001 , PMID 19900747 .
- ↑ Dimethyl Sulfoxide (DMSO) Solubility Data. (PDF) Gaylord Chemical Company, Bulletin 102, June 2014, p. 14.
- ↑ David H. Yawn: Cryopreservation. In: Britannica Online . Retrieved May 14, 2009 .
- ↑ Derek Lowe: The Miracle Solvent . In: In the Pipeline (blog) . May 20, 2008.