Swern oxidation
The Swern oxidation is a name reaction in organic chemistry that was named after its discoverer, the American chemist Daniel Swern (1916–1982). The reaction is a mild oxidation of primary or secondary alcohols to aldehydes or ketones with dimethyl sulfoxide , oxalyl chloride and triethylamine . One advantage of the Swern oxidation compared to reactions with chromium reagents is the lower toxicity of the reagents. For the reaction it is of essential importance that the reaction temperature remains well below room temperature, since the reagent formed in situ decomposes very quickly at higher temperatures. A temperature of −40 ° C is sufficiently low for the reaction. Further oxidation to carboxylic acids in the Swern oxidation is also impossible. The main disadvantage is the very unpleasant odor of the dimethyl sulfide formed during the reaction .
Overview reaction
The Swern oxidation is an oxidation of alcohols to ketones or aldehydes . Activated dimethyl sulfoxide is used as the oxidizing agent, oxalyl chloride for activation and triethylamine as the base .
mechanism
In the first step, after a nucleophilic attack by dimethyl sulfoxide (DMSO) on the oxalyl chloride and after carbon dioxide , carbon monoxide and chloride have been split off , the active intermediate, the sulfonium ion , is formed.
Both sulfonium ions that occur in this reaction are forms of activated dimethyl sulfoxide and form the same sulfonium salt with an alcohol . After adding an alcohol , it attacks the sulfonium ion nucleophilically. With the first form of the sulfonium ion, carbon dioxide , carbon monoxide and chloride are displaced and with the second form chloride is split off.
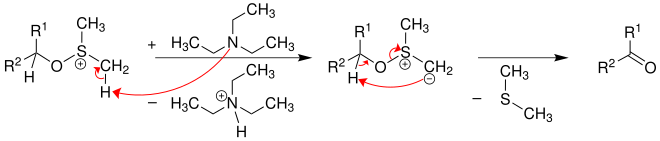
The base triethylamine finally deprotonates the sulfonium ion and with elimination of dimethyl sulfide , the aldehyde (if R 1 = H or R 2 = H) or the ketone is formed.
Individual evidence
- ↑ K. Omura, D. Swern: Oxidation of alcohols by "activated" dimethyl sulfoxide. A preparative, steric and mechanistic study. In: Tetrahedron . 34, 1978, p. 1651, doi: 10.1016 / 0040-4020 (78) 80197-5 .
- ↑ AJ Mancuso, DS Brownfain, D. Swern: Structure of the dimethyl sulfoxide-oxalyl chloride reaction product. Oxidation of heteroaromatic and diverse alcohols to carbonyl compounds. In: J. Org. Chem. 44, 1979, pp. 4148-4150, doi: 10.1021 / jo01337a028 .
- ↑ AJ Mancuso, S.-L. Huang, D. Swern: Oxidation of long-chain and related alcohols to carbonyls by dimethyl sulfoxide "activated" by oxalyl chloride. In: J. Org. Chem. 43, 1978, pp. 2480-2482, doi: 10.1021 / jo00406a041 .
- ↑ T. Laue, A. Plagens: Name and catchword reactions of organic chemistry . Teubner Verlag, 2006, ISBN 3-8351-0091-2 , p. 324-326 .
See also
Web links
- organic-chemie.ch: Swern oxidation (detailed mechanism)