Carbon monoxide
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
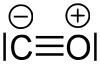
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Carbon monoxide | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | CO | |||||||||||||||||||||
| Brief description |
colorless and odorless gas |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 28.01 g mol −1 | |||||||||||||||||||||
| Physical state |
gaseous |
|||||||||||||||||||||
| density |
1.25 kg m −3 (0 ° C, 1013 mbar) |
|||||||||||||||||||||
| Melting point |
−205.07 ° C |
|||||||||||||||||||||
| boiling point |
−191.5 ° C |
|||||||||||||||||||||
| solubility |
30 mg l −1 in water (20 ° C) |
|||||||||||||||||||||
| Dipole moment | ||||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| MAK |
DFG / Switzerland: 30 ml m −3 or 35 mg m −3 |
|||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| Thermodynamic properties | ||||||||||||||||||||||
| ΔH f 0 | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Carbon monoxide (technical language carbon monoxide , commonly carbon monoxide ) is a chemical compound of carbon and oxygen with the empirical formula CO. Carbon monoxide is a colorless, odorless, tasteless and toxic gas. It arises, among other things, from the incomplete combustion of carbon-containing substances with insufficient oxygen supply. Formally, the gas is the anhydride of formic acid , but it hardly dissolves in water.
Carbon monoxide is flammable and burns with oxygen in a blue, transparent flame to form carbon dioxide . It is very reactive at elevated temperatures and reacts with various transition metals to form metal carbonyls . Carbon monoxide reacts with sulfur to form carbonyl sulphide , with alkali hydroxides to formates , with halogens such as fluorine or chlorine it reacts to form carbonyl halides such as carbonyl fluoride or phosgene . Carbon monoxide reacts with azo compounds to form isocyanates and with ammonia to form toxic formamide . As a component of the synthesis gas , it reacts in the Fischer-Tropsch synthesis to form various hydrocarbons and their oxidation products . It is also used for the synthesis of methanol and isobutanol . As a component of town gas , it was used in Germany as fuel and illuminating gas until the second half of the 20th century .
The gas is poisonous because it binds to hemoglobin more strongly than oxygen and thus prevents oxygen from being transported through the blood. The carbon monoxide poisoning is often a part of process of smoke inhalation and the mine disease after inhalation of toxic gases in mining ; it can be fatal within a short period of time.
nomenclature
In the withdrawn German standard DIN 32640 “Chemical elements and simple inorganic compounds - names and symbols” from December 1986, the spelling “carbon monoxide” with “oo” is recommended because, according to the IUPAC rules for the nomenclature of inorganic chemistry, end vowels of prefixed Greek numerals cannot be left out.
In contrast, in the 1990 edition of the IUPAC nomenclature, the spelling “carbon monoxide” is used. Regarding the use of the multiplicative prefixes it says: “The final vowels of the multiplicative prefixes are not left out, unless there are compelling linguistic reasons. Monoxide is one such exception. "
history
As early as 1000 BC Chr. Was used in the smelting of metal ores , the reducing effect of carbon monoxide in the so-called Rennöfen used, but without knowing the nature of the gas. The Greeks and Romans used it for executions . Arnaldus de Villanova described in the late 13th century the toxicity of a gas produced by burning wood; what was meant was carbon monoxide. In the early 17th century, Johan Baptista van Helmont experimented with a gas called gas carbonum , a mixture of carbon dioxide and carbon monoxide.
In 1776, the French chemist Joseph de Lassone produced carbon monoxide by heating zinc oxide with coke . He wrongly assumed that it was hydrogen. Joseph Priestley made carbon monoxide in 1799 by passing carbon dioxide over hot iron. William Cruickshank used the same procedure in 1800. He suggested the correct composition of CO. The toxic properties on dogs were studied by Claude Bernard around 1846.
At the beginning of the 19th century the need for luminous gases increased . The English coal gas industry began at this time with the development of processes for coal gasification . With the development of high-pressure processes in the chemical industry at the beginning of the 20th century, the demand for carbon monoxide-containing synthesis gases rose sharply. With the development of the Winkler generator , the Lurgi pressure gasifier and later the Koppers-Totzek reactor , large-scale coal gasification was achieved.
During the Nazi era, carbon monoxide was used for executions. During the T4 campaign , more than 70,000 people with mental and physical disabilities were systematically murdered with carbon monoxide in Germany from 1940 to 1945.
Occurrence
Atmospheric occurrences

The mean concentration of carbon monoxide in the earth's atmosphere is about 90 billionths of a volume fraction (the English term for a billionth is parts per billion ; with the addition of "v" for volume fraction, this auxiliary unit is usually abbreviated as "ppbv"). In total, the atmosphere contains around 400 mega tonnes (Mt). In the northern hemisphere , the mean concentration today is 140 ppbv; before industrialization it was around 90 ppbv. In the southern hemisphere , the mean concentration remained at its natural level of 50 ppbv.
The satellite instrument Terra's Measurements Of Pollution In The Troposphere (MOPITT) created the first global map for the CO concentration in the lower earth atmosphere. The MOPITT figure attached shows the average CO concentration from April, May and June for the years 2000 to 2004.
Carbon monoxide has an average atmospheric lifetime of about two months. The most important reaction is with hydroxyl radicals in the atmosphere, which oxidizes 2,300 Mt (2000–2800 Mt) of carbon monoxide to carbon dioxide annually. Soils contribute around 300 Mt (200–600 Mt) to CO oxidation annually. The rate of oxidation depends on the concentration of hydroxyl radicals, which increases with stronger solar radiation and higher water vapor content. In the tropics the lifespan is about a month, in temperate and northern latitudes it ranges from two months in summer to more than a year in winter. An average atmospheric lifetime of approximately one year would be required to balance the carbon monoxide concentrations between the northern and southern hemispheres. The higher anthropogenic emissions in the northern hemisphere therefore lead to a significant difference in concentration between the hemispheres. Due to weather influences such as the Indian monsoon and emissions from metropolitan areas, there are considerable regional differences, for example in East Asia .
Worldwide, around 2500 megatons (2000–2900 Mt) of carbon monoxide are released into the atmosphere or are formed there every year. Around half of this is caused by incomplete combustion of fossil fuels or biomass , including forest and bush fires. Another large proportion is only formed in the atmosphere through the oxidation of hydrocarbons such as methane or other volatile organic compounds . In total, about 60% of carbon monoxide is caused by humans, the rest is of natural origin.
Information on the global emission rates are based on estimates and are subject to uncertainty factors. Carbon monoxide emissions from traffic are given as 500 Mt (300–600 Mt), stationary plants for generating energy from fossil fuels emit 100 Mt (80–200 Mt), biomass combustion 600 Mt (300–800 Mt). The oxidation of methane produces 600 Mt (400–800 Mt), the degradation of other volatile organic compounds 500 Mt (300–700 Mt). Other biogenic sources release around 200 Mt (100–400 Mt), soils around 30 Mt and the oceans around 10 Mt annually.
Soils can be both sources and sinks for carbon monoxide. In arid regions , soils give off carbon monoxide, presumably through the abiotic decomposition of organic matter under the influence of light and high temperatures. Outside the arid regions, the predominance of degradation by prokaryotes , depending on the oxygen and water content of the soil, is either through reduction to methane ( methane-forming agent ) or through oxidation to carbon dioxide.
Oceans are oversaturated in terms of carbon monoxide . In the Atlantic Ocean , the concentration is about ten to forty times the atmospheric equilibrium concentration, with biological sources being assumed to be responsible. The oceans are therefore a source of atmospheric carbon monoxide, the proportion of which is estimated at 5 to 29% of the amount released by combustion.
A concentration of 3700 ppm ( parts per million ) carbon monoxide was measured in the gases of Hawaiian volcanoes . Volcanoes play no role in the global balance.
The main infrared absorption band of carbon monoxide is at a wave number of 2143 cm −1 ( wavelength about 4.67 µm ) and thus far from the spectral maximum of the earth's infrared radiation , which is at a wave number of about 1000 cm −1 (10 µm). Carbon monoxide therefore makes little contribution to the greenhouse effect through direct absorption . In the troposphere , the oxidation of hydrocarbons such as methane to carbon dioxide also takes place via hydroxyl radicals. Carbon monoxide is also formed as an intermediate product in methane oxidation. The effect of carbon monoxide on the greenhouse effect lies in its importance as a sink for these hydroxyl radicals, which are therefore no longer available for converting direct greenhouse gases such as methane. Also, methanol , methane according to the second most abundant organic gas in the atmosphere, which in concentrations of 0.1 to 10 ppbv occurs, is a significant source of atmospheric for carbon monoxide, wherein a major part of the methanol is emitted from plants.
In urban environments , untreated car exhaust emissions can range from 100 to 200 ppm. The concentration of carbon monoxide in the undiluted exhaust gas of an automobile without a catalytic converter is about 7000 ppm. In vehicles with a three-way catalytic converter and correctly set lambda probe control, carbon monoxide is ideally used to reduce nitrogen oxides , whereby it itself is oxidized to carbon dioxide. Due to high exhaust gas temperatures and the associated adjustment of the Boudouard equilibrium , exhaust gases from passenger cars with gasoline engines always contain a certain amount of carbon monoxide. A limit value of 1000 ppm is specified in the emission standards Euro 5 and 6 , for vehicles with diesel engines the limit value is 500 ppm. In the United States, more than half of tropospheric carbon monoxide emissions in 2002 were from vehicle operation, about 61 of 117 million tons. In metropolitan areas, vehicle exhaust was responsible for around 75% of all carbon monoxide emissions. Carbon monoxide emissions from these mobile sources have declined by around 5% per year since the early 1990s.
Carbon monoxide, nitrogen oxides and volatile hydrocarbon compounds are the precursor molecules for the formation of ozone (O 3 ) in the troposphere. The formation of ozone takes place via the oxidation of nitrogen monoxide (NO) to nitrogen dioxide (NO 2 ) by peroxy radicals , which are formed , for example, during the oxidation of carbon monoxide. These react very quickly with existing nitrogen monoxide to form nitrogen dioxide. By photolysis of nitrogen dioxide ozone is formed.
The distribution, sources and sinks of carbon monoxide differ between the troposphere and the stratosphere . In the stratosphere, the carbon isotope 14 C is continuously created through nuclear reactions , for example when the nitrogen isotope 14 N, which is by far the most common nitrogen isotope , is hit by a neutron. If a neutron is captured and a proton is split off, the 14 N nucleus becomes a 14 C nucleus. The resulting carbon is first oxidized to carbon monoxide. The residence time of carbon monoxide in the stratosphere depends, among other things, on the concentration of hydroxyl radicals by which it is oxidized to carbon dioxide.
Occurrence in buildings
In residential buildings , the normal concentration is 0.5 to 5 ppm, with concentrations of up to 15 ppm in the vicinity of gas burners . Carbon monoxide has a density of 96.5% of the density of air , so it is slightly lighter. If there is enough chimney draft , the carbon monoxide that is formed rises rapidly with the warm exhaust gas through the chimney if the carbon is not completely burned. If the chimney draft of a wood-burning stove is poor , carbon monoxide can enter the room air and cause poisoning. There are many reasons for the exhaust gas return flow or its insufficient removal. Wind, outdated or poorly adjusted and maintained boilers, an activated extractor hood or an activated central vacuum cleaner system can cause back pressure, which can lead to exhaust gas backflow. Leaky chimneys and stoves, incorrectly dimensioned chimneys or chimneys blocked by wasps or birds' nests , for example , can prevent sufficient exhaust gases from being extracted. Other sources of carbon monoxide in living rooms are barbecues over charcoal wood fires, or the operation of radiant heaters fired with propane gas . Also, gas stoves , emissions from wood pellets in pellet bunkers, car exhaust from attached garages and the use of with internal combustion engines operated aeration equipment and generators can be Kohlenstoffmonoxidquellen.
Tobacco smoke contains significant levels of carbon monoxide. Ten smoked cigarettes increase the concentration of about 22 ppm carbon monoxide in an unventilated, 30 m³ room.
Extraterrestrial occurrences
Carbon monoxide is also widespread in extraterrestrial space, outside of the earth and its atmosphere. It is created there when the much rarer carbon dioxide reacts with atomic and molecular hydrogen to form water and carbon monoxide. There are also a number of other formation reactions, such as the reaction of carbon cations with hydroxyl radicals with the formation of carbon monoxide and a proton .
Carbon monoxide has been detected in the photosphere of the Sun and the atmospheres of Venus , Mars , Jupiter , Saturn , Titan , Uranus, and Neptune . The carbon monoxide concentration in the Venusian atmosphere is about 50 ppm, in the Martian atmosphere 700 ppm were measured. In the outer solar system , carbon monoxide is the second most abundant component in the extremely thin atmosphere of the dwarf planet Pluto . Carbon monoxide has also been detected in the atmosphere of exoplanets .
The Giotto spacecraft measured 10% carbon monoxide in the gas emissions from Comet Halley . Carbon monoxide was also detected in the emissions of other comets such as Hyakutake , Hale-Bopp , and Schwassmann-Wachmann 1 . These observations led to the conclusion that carbon monoxide was a major source of carbon during the formation of the solar system. In the prehistoric times of the solar system, more complex organic molecules such as hydrocarbons and fatty acids were formed from carbon monoxide and hydrogen via Fischer-Tropsch-type reactions , which may be related to the origin of life.
An important emission line for carbon monoxide, which occurs interstellar in molecular clouds, is at a wavelength of 2.6 mm and is largely excited by collisions with hydrogen molecules. The infrared and radio observation of carbon monoxide in molecular clouds therefore serves for the indirect determination of the interstellar hydrogen concentration. It is assumed that the ratio of carbon monoxide and hydrogen is constant. Rotational transitions of carbon monoxide are an efficient mechanism for converting thermal energy into microwave and infrared radiation and are one of the important cooling mechanisms of molecular clouds. In the sufficiently dense areas of a molecular cloud, these energy losses facilitate the conditions for a gravitational collapse of a molecular cloud, which can lead to star formation . In its capacity as an indicator for the presence of hydrogen, carbon monoxide is one of the most studied interstellar molecules. By measuring the redshift of the rotational transitions of carbon monoxide, its distribution and kinematics within the galactic equatorial plane could be determined.
Extraction and presentation
Carbon monoxide can be obtained from numerous carbonaceous raw materials such as B. natural gas , biogas , light petrol , heavy oils , coal or biomass can be produced. If the raw material is coal or coke, first generator gas (contains about 25% CO in addition to N 2 ), water gas (CO: H 2 about 1: 1) or synthesis gas (CO: H 2 about 1: 2) is generated, which is cleaned and processed becomes. Methane as a raw material leads to cracked gas (CO: H 2 approx. 1: 3). The most important large-scale processes are coal gasification , steam reforming and the partial oxidation of hydrocarbons.
Coal gasification
Coal gasification takes place in so-called generators, which can produce generator gas, water gas or synthesis gas depending on the process control. The types of generator used are diverse and differ, among other things, in the type of coal feed, slag removal and the coals used and their grain sizes . In addition to the tapping generator , the fixed grate generator and the rotary grate generator , the Winkler generator , the Lurgi pressure gasifier and the Koppers-Totzek reactor are in use, the latter three being mainly used for the production of synthesis gas. In addition to the gases, tar and gases enriched with hydrocarbons are also obtained in smoldering generators .
Water and synthesis gas production
Coke is gasified at an elevated temperature by reacting it with water vapor and air or, preferably, oxygen. The gas composition can be determined by the process management. In alternation between air (bubble period or simply "bubbles") and water vapor (gas period or "gases"), water gas is generated, whereby only the gas generated during the reaction with water vapor is used. Operation with air serves the hot blowing the coke to incandescence , thereby providing the necessary energy for the endothermic water gas reaction. If the generators are operated continuously with air or oxygen and water, synthesis gas is the main product.
The change in the energy content of the system, the enthalpy , is expressed by the symbol , with endothermic reactions having a positive sign according to convention. The exponent zero stands for the standard conditions (100 kPa and 25 ° C). While the enthalpy indicates whether a process is exothermic or endothermic, the change in Gibbs energy during a chemical reaction is the decisive criterion for whether a conversion of the substances involved actually takes place voluntarily.
The main reaction is the endothermic heterogeneous water gas reaction:
This is coupled with the exothermic combustion of carbon:
There are also other reactions involved. The most important are the Boudouard equilibrium
and the homogeneous water gas reaction ( water gas shift reaction )
Generator gas production
Generator gas results from incomplete combustion of coke. To do this, air is passed through a layer of carbon and initially burned to carbon dioxide in excess air. The carbon dioxide then reacts with carbon to form carbon monoxide at high temperatures. The generator gas has a composition of about 70% nitrogen, 25% carbon monoxide and 4% carbon dioxide. The gas also contains hydrogen, methane and oxygen.
Steam reforming
Carbon monoxide can be prepared by treatment of natural gas or light petroleum are produced with water vapor fractions, for example from methane by
or generally after
The main product of steam reforming is hydrogen. The endothermic reaction takes place in the presence of nickel catalysts at temperatures of 700 to 1100 ° C. The reaction is reversible .
Partial oxidation of hydrocarbons
With partial oxidation , the starting material, for example heavy fuel oil, is oxidized with a limited amount of oxygen to form hydrogen and carbon monoxide. A distinction is made between thermal and catalytic partial oxidation. In the case of thermal partial oxidation, the reaction temperature is around 1200 ° C, in catalytic processes, depending on the catalyst, around 800 to 900 ° C:
Laboratory scale

In the laboratory, a steady stream of carbon monoxide can be produced by dripping formic acid into warm, concentrated sulfuric or phosphoric acid:
Since carbon monoxide is very poisonous, excess quantities must be collected with special absorbers or flamed with a flame device. Particular caution is required with mixtures of carbon monoxide and air, as these can react explosively . In addition, there is the possibility of converting carbon monoxide with copper (II) oxide or hopcalite into carbon dioxide catalytically.
Separation of the carbon monoxide
If pure gases are desired as the product, the gas mixture as described above must be separated. For many applications, on the other hand, synthesis gas is required directly; here only the ratio between carbon monoxide and hydrogen has to be adjusted as required. The carbon monoxide can be separated in the following ways:
- Reversible complexation with copper-aluminum salts at increased pressure; the carbon monoxide is released again at reduced pressure.
- Condensation at low temperatures
- Pressure swing adsorption
- Semipermeable membranes
properties
Physical Properties
Carbon monoxide is a colorless and odorless gas with a molar mass of 28.01 g / mol. At 1.25 kg / m³ it has almost the same density as air. The boiling point of −191 ° C and the melting point of −205 ° C are close to the values of isoelectronic (see below) nitrogen, which has a boiling point of −196 ° C and a melting point of −210 ° C.
The triple point at which the three phases solid, liquid and gaseous are in equilibrium is at a temperature of −205.0 ° C and a pressure of 0.154 bar . The critical temperature is −140.2 ° C, the critical pressure is 35.0 bar, the critical density is 0.301 g / cm³.
Upon cooling, carbon monoxide initially forms a solid, disordered, hexagonal β-phase, which changes into an ordered α-phase on further cooling below 61.6 K, which in the cubic crystal system with the space group P 2 1 3 (space group no.198) crystallized. At high pressures of over 52,000 bar, solid, whitish, yellowish or red, metastable polycarbonyl phases are formed, the exact structure of which is still unknown.
Carbon and oxygen are very tightly bound to one another in the carbon monoxide molecule. The dissociation energy is slightly higher than that of the very inert dinitrogen molecule , which is isoelectronic to carbon monoxide , and is 1070.3 kJ / mol. Only 946 kJ / mol are required to split the nitrogen molecule N 2 .
The mass-related calorific value is 10.1 MJ / kg, which is around 5 times less than that of methane. The volume-related heating and gross calorific value is 12,636 kJ / m³ and is thus in the range of hydrogen. The ignition temperature is 605 ° C. The ignition range is from 12.5 to 75 % by volume of carbon monoxide in air.
Molecular Properties
The bond length between the carbon and oxygen atoms is 106 pm in the solid phase and 112.8 pm in the gas phase. The distance between two given atoms is about 20 pm smaller if they are connected with a double bond instead of a single bond .
For a triple bond , the distance is a further 10 pm smaller than that of a double bond. The carbon-oxygen bond , which is about 10 μm shorter than a carbon-oxygen double bond of an organic carbonyl compound , for example in the case of formaldehyde , therefore indicates a triple bond.
Molecular structure is best described using molecular orbital theory . According to their rules, the number of resulting molecular orbitals is equal to the number of atomic orbitals involved . The eight molecular orbitals of carbon monoxide are formed from the four atomic orbitals of carbon and oxygen. The occupied molecular orbital of highest energy (HOMO) forms the antibonding σ s * orbital. In antibonding orbitals, the highest probability of the electrons being located is not between the atomic nuclei involved. As a result, the repulsion of the positively charged atomic nuclei is not shielded. The molecular orbitals are therefore called antibonding. The unoccupied lowest energy molecular orbitals (LUMO) are the antibonding π x, y * orbitals.
In its ground state, the molecule is in a singlet state , which means that there are no unpaired electrons in the molecule. In the Lewis structural formula, a positive formal charge occurs on the oxygen atom and a negative formal charge on the carbon atom:
Due to the high electronegativity difference of 1 between the binding partners, this formal charge is balanced again and carbon monoxide is therefore almost non-polar. Its dipole moment is only μ = 0.10980 D , the dipole moment being directed in such a way that the oxygen atom carries the positive charge.
Chemical properties
The formation of carbon monoxide from the elements is exothermic and is in a disproportionation equilibrium with carbon and carbon dioxide. Because this equilibrium is almost immeasurably slow at room temperature , carbon monoxide can be isolated despite the unfavorable equilibrium position - carbon monoxide is metastable . At higher temperatures, the equilibrium shifts in favor of carbon monoxide ( LeChatelier's principle ). This is used, for example, in iron production in the blast furnace process, where the gaseous carbon monoxide is a much more effective reducing agent than solid coke . In industrial processes, carbon monoxide can poison catalysts because it binds strongly to active metal centers such as nickel or iron atoms and blocks them for other reactants.
Carbon monoxide is a good and inexpensive reducing agent and is used in this function in a variety of ways. The oxidative power, however, is only weak. In organometallic chemistry , carbon monoxide is a monodentate ligand that is frequently used , and the chemistry of metal carbonyls has been well researched. It is one of the strong-field ligands and is isoelectronic to nitrogen (N 2 ) and to the ions cyanide (CN - ) and nitrosyl (NO + ). A strong metal-ligand bond is created through the development of synergetically strengthening back and forth bonds. Carbon monoxide is a strong σ donor and π acceptor . It migrates in alkyl-metal bonds of complexes to form acyl groups .
With aromatics such as benzene , carbon monoxide reacts with chlorine with aluminum chloride or copper (I) chloride catalysis in the Gattermann-Koch reaction to form benzaldehyde . Carbon monoxide reacts with strong reducing agents such as metallic potassium to form the potassium salt of hexahydroxybenzene or the potassium salt of dihydroxyacetylene .
use
Carbon monoxide is often used in the chemical industry together with hydrogen as a synthesis gas . For some applications, however, pure carbon monoxide is also required, for example for the production of metal carbonyls such as nickel tetracarbonyl or iron pentacarbonyl , for carbonylations or the production of cooking acids . The production of phosgene , acetic acid and acetic anhydride and the production of methyl formate and formic acid also require pure carbon monoxide.
Pure carbon monoxide is mainly obtained from synthesis gas through physical or chemical separation processes. The physical separation takes place by partial condensation and subsequent distillation according to the Linde process or by membrane permeation . Carbon monoxide can be separated from other components of the synthesis gas by means of copper (I) salt solutions through adsorption with complex formation.
The carbon monoxide used in the chemical industry is mostly produced on site and further processed in-house. There is also the option of delivery by tanker or in gas bottles . For the networking of chemical sites, distribution through pipelines is possible. Projects for the construction of such pipelines, such as Bayer AG's CO pipeline , are often criticized and can lead to lengthy administrative litigation.
Synthesis gas reactions
Methanol production
Methanol is a basic organic chemical and a large-scale, large-scale alcohol . The production from carbon monoxide and hydrogen proceeds according to the following gross equation:
In 2008, global methanol consumption was 45 million tons. Today, methanol is produced on an industrial scale from synthesis gas using the low or medium pressure process. The resulting raw methanol is partially contaminated with by-products. If the raw methanol is used for combustion in the energy sector, the purity of the raw methanol is sufficient. For further processing in the chemical industry, the methanol has to be worked up by distillation. Low-boiling components such as dimethyl ether are separated off in a low-boiling column. The higher-boiling fractions are separated off as bottoms in a further distillation stage in a high boiler column, with methanol being drawn off at the top.
Fischer-Tropsch synthesis
In the Fischer-Tropsch synthesis, a carbon monoxide-hydrogen mixture is converted by means of heterogeneous catalysis . The catalysts are based on the transition metals cobalt , iron , nickel and ruthenium ; porous metal oxides with large specific surfaces such as diatomaceous earth , aluminum oxide , zeolites and titanium dioxide are used as carriers .
The gas mixture is converted into hydrocarbons such as paraffins , olefins and alcohols in a build-up reaction . End products are gasoline ( synthetic gasoline ), diesel , heating oil and raw materials for the chemical industry. The reaction takes place even at atmospheric pressure and at a temperature of 160 to 200 ° C; technically, higher pressures and temperatures are used depending on the process. The synthesis proceeds according to the following reaction scheme:
- ( Alkanes )
- (n ≥ 2, alkenes )
- ( Alcohols )
Around 1.25 kg of water are produced per kilogram of fuel, and around half of the hydrogen used is used to produce it. The typical Fischer-Tropsch product contains around 10-15% liquefied gases ( propane and butane ), 50% petrol, 28% kerosene (diesel oil), 6% soft paraffin (slack paraffin) and 2% hard paraffins. The process is important for the large-scale production of gasoline and oils from coal , natural gas or biomass .
Hydroformylation
Together with hydrogen, carbon monoxide is used for the hydroformylation of olefins. The resulting products are primarily aldehydes , mostly metal carbonyl hydrides being used as catalysts in homogeneous or heterogeneous reactions.
A technically important product of hydroformylation with subsequent hydrogenation is 2-ethylhexanol . The plasticizer di- n -octyl phthalate can be produced from 2-ethylhexanol and phthalic anhydride .
Reaction with organic molecules and water
Formic acid and derivatives
Pure carbon monoxide is used for the production of formic acid from water and methanol via a methyl formate stage. Sodium methoxide is used as the catalyst . The methanol consumed in the first step is released again in the second step by hydrolysis . The first plant of this type was commissioned in 1981 and produced 100,000 tons per year. In 2012, BASF 's global production capacity using this process was 255,000 tons per year.
With sodium hydroxide , sodium formate is obtained, which is used as the raw material for the production of oxalic acid .
Monsanto and Tennessee Eastman Trials
By reacting carbon monoxide with methanol using mixed rhodium carbonyls, acetic acid is obtained in the Monsanto process . This is further processed into vinyl and cellulose acetate as well as intermediate products for the chemical industry.
Acetic anhydride is formed through the carbonylation of methyl acetate in the Tennessee-Eastman process .
Reppe chemistry
Carbon monoxide is used for carbonylation reactions in organic synthesis. Reppe chemistry is understood to mean working with acetylene under elevated pressure, metal carbonyls and hydrogen carbonyls being used as catalysts. An important reaction is hydrocarboxylation , in which acetylene is used with carbon monoxide and water or an alcohol to produce acrylic acid and acrylic acid esters:
Cooking acids
Tertiary carboxylic acids , so-called Koch acids, are formed by the Koch reaction of carbon monoxide with alcohols or alkenes and water under acid catalysis at high pressures and elevated temperatures.
The chemical industry produces around 150,000 tons of cooking acids per year. Some commonly industrially produced Koch acids are pivalic acid , 2,2-dimethylbutyric acid and 2,2-dimethylpentanoic acid .
Metal carbonyls
Carbon monoxide reacts with transition metals to form metal carbonyls . These are complex compounds in which carbon monoxide functions as a ligand . The metals occur in these compounds with an oxidation number of zero. Ludwig Mond succeeded in synthesizing the first homoleptic metal carbonyl complex, nickel tetracarbonyl , as early as 1890. In the years that followed, he succeeded in synthesizing a number of other metal carbonyls such as iron pentacarbonyl , dicobalt octacarbonyl and molybdenum hexacarbonyl . In many areas of organometallic chemistry , chemists use metal carbonyls as key components, for example to study chemical bonds and the targeted synthesis of organometallic complexes.
The metal carbonyls are also used in organic synthesis and as catalysts or catalyst precursors in the chemical industry . The carbon monoxide ligand can be replaced by other, tailor-made ligands, such as the water-soluble tri- (sodium-meta-sulfonatophenyl) -phosphine , and thus specifically adapted to the requirements of technical processes, such as the Ruhrchemie / Rhône-Poulenc process .
Phosgene production
Phosgene is produced from carbon monoxide and chlorine under the catalytic influence of activated carbon :
Phosgene is an important starting material for the production of methylene diisocyanates and toluene-2,4-diisocyanate . It reacts with diols with polycondensation to form polycarbonates .
Reducing agent
In the blast furnace it serves as a reducing agent for iron ore according to the gross formula:
The carbon monoxide is not added as a gas, but arises in the blast furnace from the combustion of coke and the subsequent Boudouard reaction, as well as from the reaction of hot coke with water, releasing carbon monoxide and hydrogen. Through the reduction of iron ores and the oxides of the iron companions manganese , silicon and phosphorus , carbon monoxide is oxidized to dioxide.
The reduced iron absorbs carbon from the carbon monoxide and becomes Fe 3 C, the so-called cementite , which causes the melting point to drop from 1538 ° C to around 1300 to 1400 ° C.
In the upstream of the blast furnace hot blast stoves that will in stack gas carbon monoxide contained burned:
Lunar process and carbonyl iron
The moon process uses carbon monoxide to purify the metal nickel with the formation and decomposition of nickel tetracarbonyl in a chemical transport reaction . The process was developed by Ludwig Mond in 1890 . The process is suitable for producing pure nickel metal from nickel oxides. In the first step, the oxides are reduced to the metal with hydrogen and then converted into the volatile metal carbonyl nickel tetracarbonyl at 40 to 60 ° C with carbon monoxide . This can be distilled off from the other ore constituents. At temperatures of 150 to 300 ° C, the metal carbonyl breaks down again into the pure metal and carbon monoxide. A related process is used to make carbonyl iron from iron pentacarbonyl .
Food technology
When treating meat and fish, an intense red meat color can be achieved through treatment with low concentrations of carbon monoxide. The binding of carbon monoxide and myoglobin creates a cherry-red carboxymyoglobin complex . This visually simulates freshness that is no longer present . The Basel canton laboratory has already proven the use of nitrite in tuna as a substitute . Carbon monoxide as a component of protective gas packaging is not permitted in the European Union.
Other uses
Adsorbed carbon monoxide is used in many ways in the characterization of heterogeneous contacts and other surfaces. In addition to determining the dispersion of active metal centers via chemisorption , studies of adsorbed carbon monoxide using surface-sensitive methods are used in industrial and basic research in semiconductor technology , fuel cell and materials research . In organometallic chemistry , the investigation of carbon monoxide ligands by means of infrared spectroscopy provides a wide range of information, for example about the binding modes of carbon monoxide, the complex geometry and the charge of the complex. In the case of heteroleptic metal carbonyls , infrared spectroscopy also provides information about the properties and bonding conditions of the ligand trans to the carbon monoxide .
In fur farms , carbon monoxide and dioxide are used to suffocate the mink so that the fur is damaged as little as possible.
City gas , which in the coking of coal was incurred, consisted of about 9 to 10% of carbon monoxide. About 350 m³ of raw gas were obtained per ton of coal through coking. After gas scrubbing, this was stored in gasometers and odorized with diphosphane before it was released into the gas network. The town gas was initially used for street lighting by means of gas lamps, hence the name illuminating gas . From around 1910, it was used for cooking and heating purposes in private homes. In the 1970s, the town gas was largely replaced by the non-toxic natural gas .
During the Nazi era, carbon monoxide was used for the first time in the mass killings of Action T4 in the context of euthanasia and “killing of unworthy life”, in which mainly mentally ill people were murdered. In some NS extermination camps, for example in the Majdanek concentration camp , carbon monoxide was later used for mass killing in gas chambers or gas vans (either as a component of combustion engine exhaust gases or as pure CO from pressurized gas cylinders).
toxicity
General

Carbon monoxide is a dangerous breath poison because it is easily absorbed through the lungs. Since the gas is colorless, odorless, tasteless and non- irritating , it is hardly noticeable. The individual tolerance level varies. Over the years there have been repeated spectacular accidents with carbon monoxide, such as the Balvano railway accident (1944) or the Kellogg mine accident (1972), some of which resulted in hundreds of deaths.
When it has entered the bloodstream via the lungs, it binds to the central iron atom of hemoglobin , thereby impeding the transport of oxygen in the blood, which can lead to death from asphyxiation . Symptoms of mild poisoning include headache , dizziness, and flu-like symptoms. Higher doses are significantly toxic to the central nervous system and the heart. Carbon monoxide is the cause of more than half of all fatal poisonings worldwide. In the United States , between 1979 and 1988, over 56,000 people died of carbon monoxide poisoning, with more than 25,000 cases of suicidal poisoning, according to a study . Over 15,000 cases were fire-related and more than 11,000 cases were accidental, non-fire-related deaths. The number of unintentional poisoning through automobile exhaust fumes is falling sharply due to the widespread use of three-way catalytic converters . Nonetheless, non-fire carbon monoxide poisoning resulted in approximately 15,000 hospital emergency room treatments and 500 deaths in 2011. In Germany there has been an obligation to report carbon monoxide poisoning since August 1, 1990. From this reference date to December 31, 2008, the Federal Institute for Risk Assessment received over 57,000 medical reports on poisoning or suspected cases, i.e. around 3000 per year.
effect
The percentage of hemoglobin covered with carbon monoxide in the blood is also abbreviated as COHb (carbon monoxide hemoglobin) . According to older studies, healthy adults are not at risk even with continuous exposure of eight hours a day at concentrations of up to 115 ppm ; there are only concentrations of 4% COHb in non-smokers and 7.6% in smokers . However, recent studies show that in risk groups with cardiovascular diseases , stress levels of 2.7% or more can intensify the symptoms of the disease. With higher chronic exposure above 150 to 300 ppm, dizziness, sleepiness, nausea and vomiting occur. For acutely lethal quantities of the gas ( LC 50 ) see info box (above). Deafness is increased by up to 50% with CO exposure. Other sources, which refer to more recent studies, assume, however, that values of 2–5% COHb already lead to the first symptoms. For example, 2% COHb disturb the sense of time , barely noticeably . With COHb values below 20% fatigue, headaches , palpitations and visual disturbances occur. In the 20 to 30% range, symptoms such as drowsiness , dizziness, and muscle weakness are known. In the range of 30 to 50% COHb, nausea , vomiting , concentration disorders , ringing in the ears , loss of consciousness and circulatory collapse occur. The skin turns pink. From a COHb value of 50%, deep unconsciousness occurs, accompanied by cramps and breathing disorders. There is then acute danger to life. Death occurs with COHb levels of 60 to 70%. About 85% of the inhaled carbon monoxide is bound in the blood, the remaining 15% is bound in the myoglobin as cherry-red carboxymyoglobin .
The individual carbon monoxide tolerance is influenced by various factors, such as the activity performed, the breathing rate , previous damage or diseases ( blood circulation , anemia or sickle cell anemia ). Other factors are atmospheric pressure or basal metabolic rate .
Carbon monoxide binds about 250 to 325 times more to the red blood pigment hemoglobin than oxygen , at a Kohlenstoffmonoxidanteil of 0.1% in the breathing air so about half of the red blood cells is disabled. The poisonous effect of carbon monoxide is reduced by the protein environment of the heme in the hemoglobin, so carbon monoxide binds to an unhindered heme about 26,000 times more strongly than oxygen. The reason is assumed that there is not enough space in the enzyme pocket to allow the linear Fe — CO geometry preferred by carbon monoxide, while the angled coordination preferred by dioxygen is not hindered. With an air content of around 0.5% by volume, death occurs within a few minutes. The elimination half-life of carbon monoxide from the blood is 2 to 6.5 hours, depending on the amount of carbon monoxide absorbed and the person's ventilation rate.
Consequential damage
In addition to the acute poisoning, there are consequential damages. Carbon monoxide has serious negative effects on fetal development. Chronic exposure to low levels of carbon monoxide can lead to depression . On average, exposure of more than 100 ppm is considered hazardous to health. The occupational exposure limit (OEL - previously: MAK value) is 30 ppm. Carbon monoxide can reduce life expectancy through damage to the heart. Occupational carbon monoxide poisoning is recognized as an occupational disease . Between 2005 and 2008, according to reports from the Federal Ministry of Labor and Social Affairs, around 126 carbon monoxide diseases were reported each year.
Diagnosis
By binding the carbon monoxide to hemoglobin, conventional pulse oximeters are deceived and incorrectly indicate high rates of oxygen saturation. With newer 7-wavelength pulse oximeters, however, the CO-saturated fraction of hemoglobin can also be detected. The outward signs of carbon monoxide poisoning are cherry-red mucous membranes . According to more recent studies with a high number of cases (231 patients), this clinical sign is rarely encountered, especially in milder forms of poisoning. The color is a result of the deep red hemoglobin-carbon monoxide charge-transfer complexes . Furthermore, the death spots (livores) that appear on the corpse after death can also be colored bright red by this mechanism and thus give an indication of carbon monoxide poisoning.
therapy
Patients with severe carbon monoxide poisoning are generally intubated and ventilated with positive end-expiratory pressure ( PEEP ) and 100% oxygen . Due to the significantly increased oxygen supply, the carbon monoxide is displaced by the hemoglobin. A hyperbaric oxygenation may be considered.
The half-life with which the increased CO concentration in the blood (CO- Hb value) falls through exhalation in fresh air is about 3–8 hours. The half-life can be reduced to up to 45 minutes through forced oxygen therapy (hyperventilation with pure oxygen using orotracheal intubation).
Biological importance
human
In humans, the proportion of hemoglobin COHb in the blood, which is coated with carbon monoxide, is between 0.7 and 1.1% in the venous blood, of which about 0.5% is produced endogenously . An increased carbon monoxide level in the cells leads to an up to 1000-fold increased release of the glycoprotein erythropoietin (EPO) .
Messenger substance
The enzyme heme oxygenase builds heme compounds consisting mainly of hemoglobin derived, with the release of carbon monoxide from. This enzyme and a guanylate cyclase , which is regulated by carbon monoxide, could be detected in the olfactory center of the human brain and in the olfactory bulb . According to this, carbon monoxide could serve as a gaseous messenger substance (see gasotransmitter ) for the sense of smell. The activated guanylate cyclase releases the secondary messenger substance cGMP . In addition to carbon monoxide, cGMP and cAMP , nitric oxide is also considered a second messenger . Both nitrogen and carbon monoxide, as extremely low-molecular, water-soluble gases, can penetrate biomembranes very quickly and relatively unhindered and therefore serve as neurotransmitters in the transmission of information from the primary or sensory to the secondary or long-term memory through the limbic system .
Anti-inflammatory properties
Carbon monoxide has an anti-inflammatory effect in chronic intestinal inflammation, which explains the previously puzzling fact that smokers are much less likely to develop ulcerative colitis than non-smokers. Even after lung transplants, low-dose inhalation of carbon monoxide prevents damage from ischemia or reperfusion . If kidneys that are intended for transplantation are kept in a solution that contains carbon monoxide in low concentrations, the otherwise observed increase in free heme and decrease in cytochrome P450 is inhibited, thus reducing cell-damaging lipid peroxidation . Favorable effects of carbon monoxide have also been described in animal models of septic shock , intestinal obstruction (ileus) and arteriosclerosis . In women with gestational hypertension and pregnancy poisoning (preeclampsia) , the carbon monoxide concentration in the exhaled air is reduced. Women who smoke have a reduced risk of developing preeclampsia. Drugs that can transport and release controlled amounts of carbon monoxide are under development.
Anaerobic respiration in archaea
In methane formation ( methanogenesis ), carbon monoxide is used by a few archaea as a substrate for anaerobic respiration . So Methanothermobacter thermoautotrophicus and Methanosarcina barkeri form three molecules of CO 2 and one molecule of methane from four molecules of CO , according to:
Also Methanosarcina acetivorans may use CO as a substrate, wherein parallel acetate and formate are formed. This type of acetogenesis in methanogens is called carboxidotrophic acetogenesis .
Energy and carbon source of carbon monoxide utilizing bacteria
| Physiological group (not all can grow with CO, green: aerobic, red: anaerobic) | examined representative | Reaction equation |
|---|---|---|
| Carboxidotrophic bacteria | Oligotropha carboxidovorans | 2 CO + O 2 → 2 CO 2 |
| Hydrogenogenic bacteria | Carboxydothermus hydrogenoformans | CO + H 2 O → CO 2 + H 2 |
| Acetogenic bacteria |
Moorella thermoacetica (formerly known as Clostridium thermoaceticum ) |
4 CO + 2 H 2 O → CH 3 COOH + 2 CO 2 |
| Phototrophic bacteria |
Rhodospirillum rubrum (compare Winogradsky column ) |
CO + H 2 O → CO 2 + H 2 |
| Sulfidogenic bacteria | Desulfovibrio vulgaris | 4 CO + H 2 SO 4 → 4 CO 2 + H 2 S |
Few bacteria are able to use carbon monoxide as a substrate. They use this to build up organic compounds ( carbon dioxide assimilation ) and to generate energy. The aerobically living carboxidobacteria are among the bacteria that utilize carbon monoxide . They are facultatively chemolithoautotrophic , which means that they live on the oxidation of inorganic substances. Carboxidobacteria oxidize carbon monoxide in the presence of oxygen to carbon dioxide, according to:
Alternatively, some species can use nitrate (NO 3 - ) instead of oxygen ( denitrification ), this is done with the exclusion of oxygen ( anaerobic ). A well-studied aerobic CO oxidizer is Oligotropha carboxidovorans . Carboxidobacteria thus probably counteract air pollution of the atmosphere with CO.
There are also a number of strictly anaerobic chemolitotrophs that oxidize CO in the absence of oxygen. This is what happens with Carboxydothermus hydrogenoformans according to:
The key enzyme in both types is carbon monoxide dehydrogenase (CODH). The aerobic CODH contains copper and molybdenum , while the CODH of the strictly anaerobic bacteria uses iron and nickel .
proof
Several types of carbon monoxide sensors are known. They are based on opto-chemical detection, infrared or thermal conductivity measurements , heat effect measurements , electrochemical processes or on semiconductor basis . The simplest design has optochemical sensors that indicate the change in color of a chemical when it comes into contact with carbon monoxide. The sensors are simple and inexpensive, but the carbon monoxide display is more qualitative. The formation of metal carbonyls serves as an optochemical display. A more precise measurement of the carbon monoxide concentration can be achieved with electrochemical sensors or sensors based on semiconductors. Nondispersive infrared sensors are widely used . Today there are portable and stationary electronic sensors ( gas warning devices ) on the market which allow the detection of carbon monoxide in the range of 20 to 2000 ppm in the room air. An alarm usually occurs on the basis of a concentration-time function so that false alarms, for example from cigarette smoke, are avoided as far as possible.
Diiodopentoxide I 2 O 5 is also used as a precise detection reagent , which is quantitatively reduced to elemental iodine I 2 in a U-tube at higher temperatures (approx. 80-160 ° C) in the presence of carbon monoxide , with simultaneous formation of carbon dioxide CO 2 :
The color change is also suitable for detection using test tubes . The CO content of the gas can be determined by back- titrating the iodine with thiosulfate S 2 O 3 2− ( iodometry ). Alfred Ditte researched this process with diiodine pentoxide as early as 1870. Hydrogen - even in higher concentrations - only minimally disturbs the detection accuracy of this method. The simultaneous quantitative determination of the converted carbon dioxide (determination by conductivity or by precipitation of a barium hydroxide solution ) makes the measurement very precise.
Carbon monoxide reacts with aqueous palladium salt solutions even at room temperature . In this process, Pd 2+ salts are reduced to metallic palladium, with carbon monoxide being oxidized to carbon dioxide. The precipitation of the finely divided metallic palladium colors the sample solution dark and thus indicates carbon monoxide.
literature
- AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , pp. 896-900.
- S1 guideline work under the influence of carbon monoxide (carbon monoxide) of the German Society for Occupational Medicine and Environmental Medicine. In: AWMF online (as of 2011)
- Louis Lewin : The History of Carbon Oxide Poisoning. In: Sudhoffs Archiv 3, 1910, pp. 1–35.
Web links
Individual evidence
- ↑ a b c d e f g h i j k l m n o p q r Entry on carbon monoxide in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ↑ a b David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Dipole Moments, pp. 9-53.
- ↑ Entry on carbon monoxide in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Schweizerische Unfallversicherungsanstalt (Suva): Limit values - current MAK and BAT values (search for 630-08-0 or carbon monoxide ), accessed on November 2, 2015.
- ^ Thermodynamic data of CO. National Institute for Standards and Technology, March 2013, archived from the original on February 2, 2015 ; accessed on August 16, 2014 .
- ^ IUPAC: Nomenclature of Inorganic Chemistry. German edition of the recommendations 1990. VCH, Weinheim 1994, ISBN 3-527-25713-6 , p. 76.
- ↑ Ivan Blumenthal: Carbon monoxide poisoning. In: Journal of the Royal Society of Medicine. Volume 94, No. 6, June 2001, pp. 270-272, PMC 1281520 (free full text).
- ↑ John Hrastar: liquid natural gas in the United States: A History. Mcfarland & Co Inc., 2014, ISBN 978-0-7864-7859-0 , p. 32.
- ^ RE Schofield: The Enlightened Joseph Priestley. A Study of His Life and Work from 1773 to 1804. Pennsylvania State University Press, 2004, ISBN 978-0-271-03625-0 , p. 103.
- ^ Guy H. Neild: William Cruickshank (FRS - 1802): Clinical chemist. (PDF; 1.7 MB) Retrieved July 26, 2014 .
- ^ Henry Friedlander: The Origins of Nazi Genocide: From Euthanasia to the Final Solution . The University of North Carolina Press, 1997, ISBN 978-0-8078-4675-9 , pp. 123 .
- ↑ a b c M. AK Khalil, RA Rasmussen: The Global Cycle of Carbon Monoxide: Trends and Mass Balance . In: Chemosphere . tape 20 , 1990, pp. 227-242 (English).
- ↑ a b c d M. AK Khalil, JP Pinto, MJ Shearer: Atmospheric carbon monoxide . In: Chemosphere: Global Change Science . tape 1 , p. xi – xiii (English).
- ↑ a b Terra Turns Five: How Terra Tracks Pollution. In: earthobservatory.nasa.gov. March 11, 2011, accessed January 8, 2015 .
- ↑ a b c d e Integrated Science Assessment for Carbon Monoxide. EPO, 2010, accessed August 29, 2014 .
- ↑ Sachin D. Ghude, G. Beig: Satellite observed regional distribution of tropospheric nitrogen dioxide (NO2) and carbon monoxide (CO) over the Indian sub-continent. (PDF) Retrieved September 21, 2014 (English).
- ↑ O. Badr, SD Probert: Sinks and Environmental Impacts for Atmospheric Carbon Monoxide. In: Applied Energy. Volume 50, 1995, pp. 339-372.
- ↑ W. Seiler, C. Junge: Carbon monoxide in the atmosphere. In: Journal of Geophysical Research. Volume 75, 1970, pp. 2217-2226, doi: 10.1029 / JC075i012p02217 .
- ↑ JW Swinnerton, VJ Linnenbom, RA Lamontagne: The Ocean: A Natural Source of Carbon Monoxide. In: Science. Volume 167, 1970, pp. 984-986, doi: 10.1126 / science.167.3920.984 .
- ↑ Detlev Möller: Air: chemistry, physics, biology, cleanliness, law. Verlag de Gruyter, 2003, ISBN 3-11-016431-0 , p. 18.
- ↑ JC McConnell, MB McElroy, SC Wofsy: Natural Sources of Atmospheric CO. In: Nature. Volume 233, 1971, pp. 187-188, doi: 10.1038 / 233187a0 .
- ↑ Integrated Science Assessment for Carbon Monoxide. US Environmental Protection Agency, January 2010, accessed August 16, 2014 .
- ↑ Robert C. MacDonald, Ray Fall: Detection of substantial emissions of methanol from plants to the atmosphere. In: Atmospheric Environment. Part A. General Topics. Volume 27, No. 11, 1993, pp. 1709-1713, doi: 10.1016 / 0960-1686 (93) 90233-O .
- ^ Siegfried Fred Singer: The Changing Global Environment . D. Reidel Publishing Company , Dordrecht 1975, ISBN 90-277-0385-X , pp. 90 ( limited preview in Google Book search).
- ↑ a b Tom Gosink: What Do Carbon Monoxide Levels Mean? In: Alaska Science Forum. Geophysical Institute, University of Alaska Fairbanks, January 28, 1983, archived from the original on December 25, 2008 ; Retrieved December 1, 2007 .
- ↑ Dusan Gruden: Environmental protection in the automobile industry: engine, fuels, recycling Hardcover. Vieweg + Teubner Verlag, 2008, ISBN 978-3-8348-0404-4 , p. 127.
- ↑ Reducing pollutant emissions from light vehicles. In: Regulation (EC) No. 715/2007 of the European Parliament and of the Council of June 20, 2007 on the type approval of motor vehicles with regard to emissions from light passenger cars and commercial vehicles (Euro 5 and Euro 6) and on access to repair and Maintenance information for vehicles. European Union, March 29, 2013, accessed August 11, 2014 .
- ↑ Katja Petzold: On the climatology of nitrogen oxides, ozone and carbon monoxide in the troposphere: an analysis of the MOZAIC data set. Reports from Forschungszentrum Jülich, 4327, ISSN 0944-2952 , p. 6.
- ↑ Horst Fischer, Heiko Bozem, Jos Lelieveld: The photochemical production of ozone in the troposphere. Max Planck Institute for Chemistry, 2011, accessed on August 29, 2014 .
- ↑ B. Weinstock, H. Niki: Carbon Monoxide Balance in Nature. In: Science. Volume 176, 1972, pp. 290-292, doi: 10.1126 / science.176.4032.290 .
- ↑ Methods and Standards for Environmental Measurement: Proceedings of the 8th Materials Research Symposium Held at the National Bureau of Standards. Gaithersburg, Maryland, September 20-24, 1976, Issue 464, p. 440.
- ↑ Claus Bliefert: Environmental Chemistry . 3. Edition. Wiley-VCH, Weinheim 2002, ISBN 3-527-30374-X , pp. 9 ( limited preview in Google Book search).
- ↑ Danger from the chimney. In: Berliner Kurier. November 15, 2006, accessed August 17, 2014 .
- ↑ a b Green W: An Introduction to Indoor Air Quality: Carbon Monoxide (CO). United States Environmental Protection Agency, accessed December 16, 2008 .
- ^ A. Hahn, K. Begemann, R. Burger, M. Friedemann, J. Hillebrand, H. Meyer, R. Kolbusa, M. Gessner: Ärztliche Mitteilungen bei Vergiftungen 2008 . Ed .: Press office of the Federal Institute for Risk Assessment. Berlin 2010, ISBN 3-938163-54-2 , p. 58 .
- ↑ Avoid poisonous gases from the pellet bunker. Federal Institute for Risk Assessment, July 17, 2014, accessed on August 12, 2014 .
- ^ Sabine Sickinger, Stefan Sellmeier, Oliver Meisenberg, Sebastian Schöttner: Carbon monoxide poisoning by ventilation devices? In: fire protection . No. 7 , 2011, p. 538-540 .
- ^ Queensland Health Smoking Management Policy. (No longer available online.) QH, archived from the original on November 15, 2014 ; Retrieved March 1, 2010 .
- ↑ Toni Fischer and a .: Air pollution from tobacco smoke in restaurants . In: ETH Zurich (Ed.): International Archives of Occupational and Environmental Health . tape 41 , 1978, p. 267–280 ( ucsf.edu - ethz.ch (PDF) abstract [accessed on August 26, 2015]).
- ↑ D. Talbi, E. Herbst: The gas-phase destruction of interstellar carbon dioxide: Calculations on the reactions between CO 2 and H 2 and between CO 2 and H . In: Astronomy and Astrophysics . tape 386 , no. 3 , 2002, p. 1139-1142 , doi : 10.1051 / 0004-6361: 20020312 .
- ↑ a b M. Oppenheimer, A. Dalgarno: The formation of carbon monoxide and the thermal balance in interstellar clouds. In: The Astrophysical Journal. Volume 200, 1975, pp. 419-425, bibcode : 1975ApJ ... 200..419O . doi: 10.1086 / 153805 .
- ^ TR Ayers: Thermal Bifurcation of the Outer Photosphere. In: Solar Photosphere: Structure, Convection, and Magnetic Fields. In: Proceedings of the 138th Symposium of the International Astronomical Union. Springer, 1989, ISBN 0-7923-0529-9 , pp. 23-28.
- ^ Gordon Newkirk Jr .: Carbon Monoxide in the Solar Atmosphere. In: The Astrophysical Journal. Volume 125, 1957, p. 571, bibcode : 2005A & A ... 438.1043W . doi: 10.1051 / 0004-6361: 20042550 .
- ↑ Pierre Connes et al. a .: Carbon monoxide in the Venus atmosphere. In: The Astrophysical Journal. Volume 152, 1968, pp. 731-743, bibcode : 1968ApJ ... 152..731C . doi: 10.1086 / 149590 .
- ^ Lewis D. Kaplan, Janine Connes, Pierre Connes: Carbon monoxide in the Martian atmosphere. In: The Astrophysical Journal. Volume 157, 1969, p. L187, bibcode : 1969ApJ ... 157L.187K ; doi: 10.1086 / 180416 .
- ^ R. Beer: Detection of carbon monoxide in Jupiter. In: The Astrophysical Journal. Volume 200, 1975, pp. L167-L169, bibcode : 1975ApJ ... 200L.167B . doi: 10.1086 / 181923 .
- ↑ Keith S. Noll et al. a .: Detection of carbon monoxide in Saturn. In: The Astrophysical Journal. Volume 309, 1986, pp. L91-L94, bibcode : 1986ApJ ... 309L..91N . doi: 10.1086 / 184768 .
- ↑ MA Lopez-Valverde, E. Lellouch, A. Coustenis: Carbon monoxide fluorescence from Titan's atmosphere. In: Icarus. Volume 175, 2005, pp. 503-521, doi: 10.1016 / j.icarus.2004.12.015 .
- ↑ A. Marten et al. a .: First observations of CO and HCN on Neptune and Uranus at millimeter wavelengths and the implications for atmospheric chemistry. In: The Astrophysical Journal. Volume 406, 1993, pp. 285-297, bibcode : 1993ApJ ... 406..285M . doi: 10.1086 / 172440 .
- ↑ K. Lodders: The Origin of Carbon Monoxide in Neptune's Atmosphere. In: Icarus. Volume 112, 1994, pp. 368-375, doi: 10.1006 / icar.1994.1190 .
- ↑ Detlev Möller: Air: chemistry, physics, biology, cleanliness, law. Verlag de Gruyter, 2003, ISBN 3-11-016431-0 , p. 17.
- ↑ Ken Croswell: Nitrogen in Pluto's Atmosphere. In: KenCroswell.com. June 20, 1992, accessed June 28, 2013 .
- ↑ QM Konopacky, TS Barman, BA Macintosh, C. Marois: Detection of Carbon Monoxide and Water Absorption Lines in an Exoplanet Atmosphere. In: Science. Volume 339, 2013, pp. 1398-1401, doi: 10.1126 / science.1232003 .
- ↑ Halley: Flyby March 13, 1986. In: ESA.int. ESA, accessed June 28, 2013 .
- ↑ Michael A. DiSanti: Evidence for a dominant native source of carbon monoxide in Comet C / 1996 B2 (Hyakutake). In: Journal of Geophysical Research. Volume 108, 2003, p. 5061, doi: 10.1029 / 2002JE001961 .
- ↑ Michael A. DiSanti, Michael J. Mumma, Neil Dello Russo, Karen Magee-Sauer, Robert Novak, Terrence W. Rettig: Identification of two sources of carbon monoxide in comet Hale – Bopp. In: Nature. Volume 399, 1999, pp. 662-665, doi: 10.1038 / 21378 .
- ^ Matthew C. Senay, David Jewitt: Coma formation driven by carbon monoxide release from comet Schwassmann-Wachmann 1. In: Nature. Volume 371, pp. 229-230, doi: 10.1038 / 371229a0 .
- ↑ Damon P. Simonelli, James B. Pollack, Christopher P. Mckay, Ray T. Reynolds, Audrey L. Summers: The carbon budget in the outer solar nebula. In: Icarus. Volume 82, 1989, pp. 1-35, doi: 10.1016 / 0019-1035 (89) 90020-1 .
- ↑ Muriel Gargaud, Ricardo Amils: Encyclopedia of Astrobiology. Volume 1, Springer, 2011, ISBN 978-3-642-11271-3 , pp. 245–246 ( limited preview in Google book search).
- ↑ Craig Kulesa: Overview: Molecular Astrophysics and Star Formation. October 17, 1999, accessed July 27, 2014 .
- ↑ Gerrit L. Verschuur: Interstellar Matters: Essays On Curiosity And Astronomical Discovery. Springer, 2013, ISBN 978-3-7643-6696-4 , p. 247.
- ^ WB Burton, MA Gordon: Carbon monoxide in the Galaxy. III. The overall nature of its distribution in the equatorial plane. In: Astronomy and Astrophysics. Volume 63, 1978, pp. 7-27, bibcode : 1978A & A .... 63 .... 7B .
- ^ Klaus Weissermel , Hans-Jürgen Arpe : Industrial Organic Chemistry . 3. Edition. VCH, Weinheim 1997, ISBN 3-527-28838-4 , pp. 15 .
- ^ Karl Heinz Büchel, Hans-Heinrich Moretto, Dietmar Werner: Industrial Inorganic Chemistry . 2nd Edition. Wiley-VCH, Weinheim 2000, ISBN 978-3-527-29849-5 , pp. 15 .
- ↑ a b Jürgen Bierhals: Carbon Monoxide . In: Ullmann's Encyclopedia of Industrial Chemistry . 6th edition. Wiley-VCH, Weinheim 2002, ISBN 978-3-527-30385-4 .
- ^ A b Karl-Heinz Schmidt, Ingo Romey, Fritz Mensch: Coal, petroleum, natural gas: chemistry and technology. Vogel Verlag, 1981, ISBN 3-8023-0684-8 , pp. 56-64.
- ^ A b c d Karl-Heinz Schmidt, Ingo Romey, Fritz Mensch: Coal, petroleum, natural gas: chemistry and technology. Vogel Verlag, 1981, ISBN 3-8023-0684-8 , p. 65.
- ↑ a b c d e A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 898.
- ^ R. Conrad: New chemical processes. In: Chemical Engineer Technology - CIT. Volume 42, 1970, pp. 1555-1568, doi: 10.1002 / cite.330422410 .
- ^ Gaurav Nahar, Valerie Dupont: Hydrogen production from simple alkanes and oxygenated hydrocarbons over ceria - zirconia supported catalysts: Review. In: Renewable and Sustainable Energy Reviews . Volume 32, 2014, pp. 777-796, doi: 10.1016 / j.rser.2013.12.040 .
- ↑ Ekkehard Fluck, Carl Mahr: Inorganic basic internship . 6th edition. VCH, Weinheim 1985, ISBN 3-527-26032-3 , pp. 234 .
- ↑ Georg Brauer (Ed.), With the collaboration of Marianne Baudler a . a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume II, Ferdinand Enke, Stuttgart 1978, ISBN 3-432-87813-3 , pp. 621-622.
- ^ A b c d Karl-Heinz Schmidt, Ingo Romey, Fritz Mensch: Coal, petroleum, natural gas: chemistry and technology. Vogel Verlag, 1981, ISBN 3-8023-0684-8 , pp. 175-176.
- ^ Allen I. Katz, David Schiferl, Robert L. Mills: New phases and chemical reactions in solid carbon monoxide under pressure. In: The Journal of Physical Chemistry. Volume 88, 1984, pp. 3176-3179, doi: 10.1021 / j150659a007 .
- ↑ M. Lipp, WJ Evans, V. Garcia-Baonza, HE Lorenzana: Carbon Monoxide: Spectroscopic Characterization of the High-Pressure Polymerized Phase. In: Journal of Low Temperature Physics. Volume 111, 1998, pp. 247-256, doi: 10.1023 / A: 1022267115640 .
- ↑ N. Rademacher, L. Bayarjargal, W. Morgenroth, B. Winkler, J. Ciezak-Jenkins: Preparation and characterization of solid carbon monoxide at high pressure in the diamond anvil cell. (PDF) DESY, 2011, accessed on August 9, 2014 .
- ↑ http://www.uni-magdeburg.de/isut/TV/Download/Kapitel3_VerbrnungSS2003.pdf
- ↑ Linus Pauling: Fundamentals of chemistry. Verlag Chemie, 1969, ISBN 3-527-25392-0 , p. 149.
- ^ JE Huheey, EA Keiter, RL Keiter: Inorganic Chemistry: Principles of Structure and Reactivity. de Gruyter, 2003, ISBN 3-11-017903-2 , p. 173.
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1789.
- ↑ B. Rosenblum, A. Nethercot, C. Townes: Isotopic Mass Ratios, Magnetic Moments and the Sign of the Electric Dipole Moment in Carbon Monoxide. In: Physical Review. Volume 109, 1958, pp. 400-412, doi: 10.1103 / PhysRev.109.400 .
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 900.
- ↑ L. Gattermann, JA Koch: A synthesis of aromatic aldehydes. In: Reports of the German Chemical Society. Volume 30, 1897, pp. 1622-1624, doi: 10.1002 / cber.18970300288 .
- ↑ WA Herrmann : 100 years of metal carbonyls. A chance discovery makes history. In: Chemistry in Our Time . Volume 22, No. 4, 1988, pp. 113-122, doi: 10.1002 / ciuz.19880220402 .
- ^ A b Wilhelm Keim, Arno Behr, Günther Schmitt: Basics of industrial chemistry. Otto Salle Verlag, 1985, ISBN 3-7935-5490-2 , p. 104.
- ^ Commissioning of the Bayer carbon monoxide pipeline Dormagen-Krefeld / Uerdingen stopped for the time being. (No longer available online.) Higher Administrative Court of North Rhine-Westphalia, December 18, 2007, archived from the original on July 14, 2014 ; Retrieved July 13, 2014 .
- ^ Wilhelm Keim, Arno Behr, Günther Schmitt: Basics of industrial chemistry. Otto Salle Verlag, 1985, ISBN 3-7935-5490-2 , pp. 179-182.
- ↑ Andrei Y. Khodakov, Wei Chu, Pascal Fongarland: Advances in the Development of Novel Cobalt Catalysts for Fischer-Tropsch Synthesis of Long-Chain Hydrocarbons and Clean Fuels. In: ChemInform. Volume 38, 2007, pp. 1692-1744, doi: 10.1002 / chin.200733255 .
- ↑ PL Spath, DC Dayton: Preliminary Screening - Technical and Economic Assessment of Synthesis Gas to Fuels and Chemicals with Emphasis on the Potential for Biomass-Derived Syngas. (PDF) In: NREL / TP510-34929. National Renewable Energy Laboratory, December 2003, accessed August 16, 2014 .
- ^ A b Wilhelm Keim, Arno Behr, Günther Schmitt: Basics of industrial chemistry. Otto Salle Verlag, 1985, ISBN 3-7935-5490-2 , pp. 185-187.
- ^ A b Wilhelm Keim, Arno Behr, Günther Schmitt: Basics of industrial chemistry. Otto Salle Verlag, 1985, ISBN 3-7935-5490-2 , pp. 239-240.
- ↑ a b Tradition of Ideas: Formic Acid. December 2012, accessed July 11, 2014 .
- ^ Wilhelm Keim, Arno Behr, Günther Schmitt: Basics of industrial chemistry. Otto Salle Verlag, 1985, ISBN 3-7935-5490-2 , pp. 241-243.
- ^ Joseph R. Zoeller, Victor H. Agreda, Steven L. Cook, Norma L. Lafferty, Stanley W. Polichnowski, David M. Pond: Eastman chemical company acetic anhydride process. In: Catalysis Today . Volume 13, 1992, pp. 73-91, doi: 10.1016 / 0920-5861 (92) 80188-S .
- ↑ A. Gossauer: Structure and reactivity of biomolecules. Helvetica Chimica Acta, Zurich 2006, ISBN 3-906390-29-2 , p. 155.
- ↑ Jürgen Falbe, Helmut Bahrmann: Homogeneous Catalysis in Technology. In: Chemistry in Our Time. Volume 15, 1981, pp. 37-45, doi: 10.1002 / ciuz.19810150203 .
- ↑ L. Mond, C. Langer, F. Quincke: Action of Carbony Monoxide on Nickel. In: J. Chem. Soc. 1890, 57, pp. 749-753 ( doi: 10.1039 / CT8905700749 ).
- ^ Ludwig Mond, Heinrich Hirtz, Matthewman Dalton Cowap: Note on a Volatile Compound of Cobalt With Carbon Monoxide. In: Chem. News. Vol. 98, 1908, p. 165; Chem. Abs. Vol. 2, 1908, p. 3315.
- ↑ Dirk Steinborn: Fundamentals of organometallic complex catalysis. Teubner, Wiesbaden 2007, ISBN 978-3-8351-0088-6 , p. 83.
- ^ Wilhelm Keim, Arno Behr, Günther Schmitt: Basics of industrial chemistry. Otto Salle Verlag, 1985, ISBN 3-7935-5490-2 , p. 391.
- ^ Günther Neroth, Dieter Vollenschaar: Iron and steel. In: Wendehorst building materials. Vieweg + Teubner Verlag, 2011, ISBN 978-3-8351-0225-5 , pp. 637-702.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Heat of Combustion, pp. 5-70.
- ↑ L. Mond, C. Langer, F. Quincke: Action of Carbony Monoxide on Nickel. In: J. Chem. Soc. Volume 57, 1890, pp. 749-753, doi: 10.1039 / CT8905700749 .
- ↑ Eckard Amelingmeier u. a .: RÖMPP Lexicon Chemistry. Volume 4, 10th edition, 1996–1999, Georg Thieme Verlag, ISBN 978-3-13-200031-5 , p. 2008.
- ↑ Lower Saxony State Office for Consumer Protection and Food Safety : A fish sees red - treatment, storage and transport of fresh fish
- ↑ Health Department of the Canton of Basel-Stadt: Illegal coloring of tuna meat. In: gd.bs.ch . May 28, 2019. Retrieved May 28, 2019 .
- ↑ Ludwig Bergmann, Clemens Schaefer: Textbook of Experimental Physics, Volume 6. Solid. Gruyter, 2005, ISBN 3-11-017485-5 , pp. 335-336.
- ↑ K. Veera Reddy: Symmetry and Spectroscopy of Molecules. New Age Science Ltd., 2005, ISBN 81-224-1142-8 , p. 381.
- ^ Ordinance on the keeping of fur animals . § 6 Killing . (No longer available online.) September 29, 1998, archived from the original on July 8, 2012 ; Retrieved August 29, 2014 .
- ^ Karl-Heinz Schmidt, Ingo Romey, Fritz Mensch: Coal, petroleum, natural gas: chemistry and technology. Vogel Verlag, 1981, ISBN 3-8023-0684-8 , p. 152.
- ^ Matthias Felsch: Aktion T4, The First Phase of Euthanasia in National Socialism , Munich 2003.
- ↑ Barbara Schwindt: The Majdanek Concentration and Extermination Camp: Functional Change in the Context of the “Final Solution” , Verlag Königshausen u. Neumann, 2005, p. 161 f.
- ^ JA Raub, M. Mathieu-Nolf, NB Hampson, SR Thom: Carbon monoxide poisoning-a public health perspective . In: Toxicology . tape 145 , no. 1 , April 2000, pp. 1–14 , doi : 10.1016 / S0300-483X (99) 00217-6 , PMID 10771127 .
- ^ Maria E. Stefanidou, Constantine P. Maravelias, Artemis A. Dona, Constantine M. Pistos, Chara A. Spiliopoulou, Sotirios A. Athanaselis: Carbon Monoxide-Related Deaths in Greece. In: The American Journal of Forensic Medicine and Pathology. Volume 33, 2012, pp. 128-131, doi: 10.1097 / PAF.0b013e318252eca9 .
- ^ A b Nathaniel Cobb: Unintentional Carbon Monoxide-Related Deaths in the United States, 1979 Through 1988. In: JAMA: The Journal of the American Medical Association. Volume 266, 1991, p. 659, doi: 10.1001 / jama.1991.03470050059023 .
- ↑ Jorge A. Guzman: Carbon Monoxide Poisoning. In: Critical Care Clinics. Volume 28, 2012, pp. 537-548, doi: 10.1016 / j.ccc.2012.07.007 .
- ↑ Carbon monoxide poisoning in the interior is increasing. Federal Institute for Risk Assessment, February 3, 2010, accessed on August 9, 2014 .
- ↑ carbon monoxide. Retrieved August 29, 2014 .
- ↑ Markus Fritz: Clip and clear. 100 × environment. Bibliographisches Institut AG, Mannheim 1977, ISBN 3-411-01706-6 , p. 18.
- ↑ a b c Ingo Wirth, Hansjörg Strauch: Forensic medicine: basic knowledge for investigative practice. Verlag Kriminalistik, 2012, ISBN 978-3-7832-0021-8 , pp. 196-198.
- ^ GS Lipman: Carbon monoxide toxicity at high altitude . In: Wilderness & Environmental Medicine . tape 17 , no. 2 , 2006, p. 144-145 , PMID 16805152 .
- ↑ Environmental Health Criteria (EHC) for Carbon Monoxide , accessed November 29, 2014.
- ↑ JP Collman, JI Brauman, TR Halbert, KS Suslick: Nature of O 2 and CO Binding to Metalloporphyrins and Heme Proteins. In: Proceedings of the National Academy of Sciences (PNAS). Volume 73, No. 10, October 1976, pp. 3333-3337, PMC 431107 (free full text).
- ↑ Klaus Ellinger, Harald Genzwürker (Ed.): Course book emergency medicine. Oriented towards the nationwide curriculum additional designation emergency medicine. Deutscher Ärzte-Verlag, 2011, ISBN 978-3-7691-0613-8 , p. 620.
- ↑ Hu & Speizer : Environmental and occupational hazards. Carbon monoxide. In: Harrison's Principles of Internal Medicine. 14th edition. McGraw-Hill, New York City, p. 2533.
- ↑ List of occupational exposure limits and short-term values. BG Bau, January 2006, accessed on August 17, 2014 .
- ↑ CR Henry, D. Satran, B. Lindgren, C. Adkinson, CI Nicholson, TD Henry: Myocardial Injury and Long-term Mortality Following Moderate to Severe Carbon Monoxide Poisoning . In: JAMA . tape 295 , no. 4 , January 2006, pp. 398-402 , doi : 10.1001 / jama.295.4.398 , PMID 16434630 .
- ^ S1 guideline work under the influence of carbon monoxide (carbon monoxide) of the German Society for Occupational Medicine and Environmental Medicine. In: AWMF online (as of 2011)
- ↑ M. Coulange, A. Barthelemy, F. Hug, AL Thierry, L. De Haro: Reliability of new pulse CO-oximeter in victims of carbon monoxide poisoning. In: Undersea Hyperb Med. Volume 35, No. 2, March-April 2008, pp. 107-111, PMID 18500075 .
- ↑ AA Cevik et al. a .: Interrelation between the PSS, CO-Hb levels and in-hospital clinical course of CO poisoning. In: The International Journal of Clinical Practice. Vol. 60, No. 12, 2006, pp. 1558-1564, PMID 16918999 .
- ↑ Weaver et al. a .: Carbon monoxide poisoning: risk factors for cognitive sequelae and the role of hyperbaric oxygen. In: American Journal of Respiratory and Critical Care Medicine. Volume 176, No. 5, 2007, pp. 491-497, PMID 17496229 .
- ↑ Weaver et al. a. Hyperbaric oxygen for acute carbon monoxide poisoning. In: The New England Journal of Medicine . Volume 347, No. 14, October 2002, pp. 1057-1067, PMID 12362006 .
- ↑ Advice for smokers in pharmacies - Bern National Smoking Quit Program , 2008 ... October 16, 2014, accessed on March 30, 2017. - HWZ: 3–6 hours, sleep 4–8 hours.
- ↑ Daunderer: Clinical Toxicology - Gases: Carbon monoxide HI-4.3 33. Erg-Lfg. 1/88 (1988), accessed March 30, 2017.
- ↑ a b Cathérine Simone Gebhard: Investigations on the regulation of the carbon monoxide-related stimulation of erythropoietin secretion in the rat . Tübingen 2007, DNB 983723877 , urn : nbn: de: bsz: 21-opus-27538 (dissertation).
- ↑ carbon monoxide. In: Lexicon of Neurology. Retrieved August 29, 2014 .
- ↑ P. Weydt: Receptor-mediated calcium signals in cultured human glioma cells. Tectum Verlag , 2000, ISBN 3-8288-8137-8 , p. 14.
- ^ RF Schmidt, F. Lang: Physiology of humans. Learning and memory. Springer-Verlag , ISBN 978-3-540-32908-4 , p. 225.
- ^ PA Berg: Chronic fatigue and fibromyalgia syndrome. Springer-Verlag, 2003, ISBN 3-540-44194-8 , p. 37.
- ↑ The "good" thing about carbon monoxide - immunology: anti-inflammatory effect in the intestine. December 24, 2005, accessed August 29, 2014 .
- ^ Refaat AF Hegazi, Kavitha N. Rao, Aqila Mayle, Antonia R. Sepulveda, Leo E. Otterbein, Scott E. Plevy: Carbon monoxide ameliorates chronic murine colitis through a heme oxygenase 1-dependent pathway. In: Journal of Experimental Medicine. Volume 202, 2005, pp. 1703-1713, doi: 10.1084 / jem.20051047 .
- ↑ Atsunori Nakao, Gaetano Faleo, Hiroko Shimizu, Kiichi Nakahira, Junichi Kohmoto, Ryujiro Sugimoto, Augustine MK Choi, Kenneth R. McCurry, Toru Takahashi, Noriko Murase: Ex vivo carbon monoxide prevents cytochrome P450 degradation and ischemia // reperfusion injury of kidney grafts . In: Kidney International . tape 74 , 2008, p. 1009-1016 , PMID 11035334 .
- ↑ Atsunori Nakao, Augustine MK Choi, Noriko Murase: Protective effect of carbon monoxide in transplantation . In: Journal of Cellular and Molecular Medicine . tape 10 , no. 3 , June 1, 2006, p. 650-671 , doi : 10.1111 / j.1582-4934.2006.tb00426.x , PMID 16989726 .
- ↑ M. Baum: End-tidal carbon monoxide measurements in women with pregnancy-induced hypertension and preeclampsia . In: American Journal of Obstetrics and Gynecology . tape 183 , no. 4 , October 2000, p. 900-903 , doi : 10.1067 / mob.2000.109047 , PMID 11035334 .
- ^ S. Bainbridge, E. Sidle, G. Smith: Direct placental effects of cigarette smoke protect women from pre-eclampsia: the specific roles of carbon monoxide and antioxidant systems in the placenta . In: Medical Hypotheses . tape 64 , no. 1 , 2005, p. 17-27 , doi : 10.1016 / j.mehy.2004.06.019 , PMID 15533604 .
- ^ Roberta Foresti, Mohamed G. Bani-Hani, Roberto Motterlini: Use of carbon monoxide as a therapeutic agent: promises and challenges . In: Intensive Care Medicine . tape 34 , no. 4 , April 2008, p. 649-658 , doi : 10.1007 / s00134-008-1011-1 , PMID 18286265 .
- ^ Y. Liu, WB Whitman: Metabolic, phylogenetic, and ecological diversity of the methanogenic archaea. In: Annals of the New York Academy of Sciences. Volume 1125, March 2008, pp. 171-189, doi: 10.1196 / annals.1419.019 . PMID 18378594 . (Review).
- ↑ E. Oelgeschläger, M. Rother: Carbon monoxide-dependent energy metabolism in anaerobic bacteria and archaea. In: Archives of microbiology. Volume 190, No. 3, September 2008, pp. 257-269, doi: 10.1007 / s00203-008-0382-6 . PMID 18575848 . (Review).
- ^ W. Martin, MJ Russell: On the origin of biochemistry at an alkaline hydrothermal vent. In: Philosophical transactions of the Royal Society of London. Series B, Biological sciences. Volume 362, No. 1486, October 2007, pp. 1887-1925, doi: 10.1098 / rstb.2006.1881 . PMID 17255002 . PMC 2442388 (free full text). (Review).
- ↑ a b Lothar Gremer, Vitali Svetlitchnyi, Ortwin Meyer: Living with carbon monoxide: Biological catalysis of unusual mixed-metal centers. In BioSpektrum issue 5, 2001, 7th year. PDF (free full text).
- ↑ Georg Fuchs (Ed.): General Microbiology. Founded by Hans Günter Schlegel , 8th edition. Georg Thieme Verlag, Stuttgart, New York 2007, ISBN 978-3-13-444608-1 , p. 344.
- ↑ Carboxidobacteria. In: Compact Lexicon of Biology. Spektrum.de , accessed on September 27, 2014 .
- ↑ Steven Heylen, Johan A. Martens: Advances towards a simple chromogenic carbon monoxide detection. In: Angewandte Chemie. Volume 122, 2010, pp. 7794-7795, doi: 10.1002 / anie.201002569 .
- ↑ a b c Jürgen Fessmann, Helmut Orth: Applied chemistry and environmental technology for engineers: manual for study and operational practice. Verlag ecomed, 2002, ISBN 3-609-68352-X , pp. 177-178.
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 488.













































