Nitrosyl compounds
Nitrosyl is a part of the name of chemical compounds that contain NO + or NO - ions, the name is more common for the former. The compounds are derived from nitrogen monoxide NO.
The reduction of nitrogen monoxide results in the hyponitrite - anion NO - which is unstable at temperatures above −95 ° C :
The very easy oxidation of NO produces the nitrosyl or nitrosonium cation NO + :
Examples of nitrosyl compounds are:
- Nitrosyl halides
- Nitrosyl fluoride NOF
- Nitrosyl chloride NOCl
- Nitrosyl bromide NOBr
- Other
- Black Roussin ammonium salt NH 4 [Fe 4 S 3 (NO) 7 ]
- Nitrosyl sulfuric acid / nitrosyl hydrogen sulfate NOHSO 4
- Nitrosyl tetrafluoroborate [NO] BF 4
- Nitrosyl cyanide NOCN
- Nitrosyl azide NON 3
- Nitrosyl perchlorate NOClO 4
- Nitrosyltricarbonyltriphenylphosphine manganese (-I) [Mn (CO) 3 (NO) P (C 6 H 5 ) 3 ]
- Nitrosyl-tris (triphenylphosphane) -rhodium [(C 6 H 5 ) 3 P] 3 Rh (NO)
- Nitrosyl hexafluorophosphate NOPF 6
- Nitrosobenzene C 6 H 5 NO
Nitrosyl salts react with water to form nitrous acid
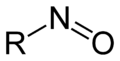
Organic compounds of the general form R 1 R 2 R 3 C-N = O, which are formally derived from the NO - ion, are referred to as nitroso compounds . The common feature of these compounds is the NO group, which is known as the nitroso group as a functional group .
If NO (i.e. nitrogen monoxide) occurs neutrally or as an anion or cation as a ligand in complex compounds , these are sometimes referred to as metal-nitrosyl complexes. The nitrosyl ion is isoelectronic to CO, N 2 or CN - , has a degree of bond of 3 and occurs as a nitrosyl radical in mononuclear complexes in a linear or angled arrangement.
swell
- ↑ Erwin Riedel ; Modern Inorganic Chemistry, 3rd Edition, pp. 663ff