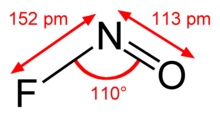
Nitrosyl fluoride
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
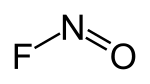
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Nitrosyl fluoride | ||||||||||||||||||
| other names |
Nitric oxide fluoride |
||||||||||||||||||
| Molecular formula | NOF | ||||||||||||||||||
| Brief description |
colorless gas |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 49.00 g mol −1 | ||||||||||||||||||
| Physical state |
gaseous |
||||||||||||||||||
| Melting point |
-132.5 ° C |
||||||||||||||||||
| boiling point |
-59.9 ° C |
||||||||||||||||||
| Vapor pressure | |||||||||||||||||||
| solubility |
Decomposes on contact with water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Nitrosyl fluoride is a chemical compound from the group of nitrosyl compounds and fluorides .
Extraction and presentation
Nitrosyl fluoride can be obtained through the following reactions:
- Reaction of nitrosyl tetrafluoroborate with sodium fluoride in vacuo at 300 ° C
- Reaction of nitrogen monoxide with fluorine at room temperature, with nitrosyl trifluoride being formed as a by-product
- Reaction of potassium fluoride with nitrogen dioxide
properties
Nitrosyl fluoride is a colorless gas that, as a liquid, is often bluish in color due to impurities. It dissolves in water with a blue color, but then quickly releases nitrogen monoxide and nitric acid . It reacts violently with glass, but is more resistant to quartz.
Individual evidence
- ↑ a b c d e Georg Brauer (Ed.), With the collaboration of Marianne Baudler u. a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume I, Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6 , p. 201.
- ^ A b c d e f Charles T. Ratcliffe and Jean´ne M. Shreeve: Nitrosyl halides - A. Nitrosyl fluoride . In: William L. Jolly (Ed.): Inorganic Syntheses . tape 11 . McGraw-Hill Book Company, Inc., 1968, p. 194-200 (English).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , p. 711.