Complex chemistry
The complex chemistry ( lat. Complexum , "surround", "hugging", "clutching") is a field of inorganic chemistry . The term coordination chemistry is generally used synonymously. Complex chemistry deals with complexes or coordination compounds that are built up from one or more central particles and one or more ligands . The central particles are mostly atoms or ions of transition metals , which can be charged or uncharged.
In contrast to conventional covalent bonds, in complexes the ligands usually contribute all electrons to the bond, instead of each reactant contributing one electron to an electron pair bond; nevertheless there are also complexes of a more covalent nature. The formation of complexes can thus be understood as an acid-base reaction in the sense of the Lewis definition , in which the ligands (bases) act as electron pair donors and the central particle (acid) as an acceptor due to gaps in its electronic configuration.
Complex compounds play an important role in various areas: in technology (for example as a catalyst, see illustration of the Grubbs catalyst on the right), in biology ( hemoglobin and chlorophyll ), in medicine ( chelation therapy ) or in analytical chemistry . Compounds with organic ligands are also the subject of organometallic chemistry . Since complexes of transition metals are sometimes very colored, these are also used as dyes or pigments ( Berlin blue ). The charge-transfer complexes show particularly intense colors , such as B. Permanganates .
For a long time chemists had no idea of the structure of coordinative compounds known as “higher order compounds”. In addition, many of the behavior of complexes could not be explained with the theories of the time, such as the stability of cobalt (III) chloride in aqueous solution when ammonia is added. For the correct interpretation of the structure and bonding relationships in complexes, the Swiss Alfred Werner received the Nobel Prize for Chemistry in 1913 as the first and for decades the only inorganic chemist .
history

Systematic research into the structure of complex compounds began in the late 19th century and is mainly characterized by the names Sophus Mads Jørgensen and Alfred Werner . Jørgensen made a name for himself with the synthesis of numerous new complexes, but was a supporter of the "chain theory" introduced by Christian W. Blomstrand . According to this theory, the ligands are lined up one behind the other and thus form chains, which makes little sense nowadays, but at least takes into account the value of the metal.
Werner, who was considered Jørgensen's opponent for decades, however, formulated a theory in 1892 that is basically still valid today and was first published the following year. The statements that can be derived from this, for example about possible stereoisomerism, could be confirmed experimentally and solidified the theory over the years. A few years after being awarded the Nobel Prize in Chemistry, Werner died in 1919.
In the decades that followed, the development of new theories about chemical bonding and new technologies such as X-ray structure analysis led to great advances in coordination chemistry. In addition, since then there have been other Nobel prizes in chemistry in the field of complex chemistry, for example in 1973 for the research of so-called sandwich complexes by Ernst Otto Fischer and Geoffrey Wilkinson , or in 2005 for the description of olefin metathesis and the development of suitable (complex) catalysts by Yves Chauvin , Robert Grubbs and Richard R. Schrock .
nomenclature
Complex formulas
The oxidation number is determined by looking at the original charge of the central atom, as if all ligands were removed while taking along the shared electron pairs. The sum of the charge contributions of the ligands and the oxidation number of the central particle (s) must give the charge of the complex.
- The coordination unit is shown in square brackets. Any charge that may be present is written as an exponent behind the square brackets. In this case the coordination unit is also referred to as a complex ion.
- The central particle is named before the ligand.
- Ligands are listed alphabetically (as opposed to previous recommendations to avoid problems with so-called non-innocent ligands ) regardless of their charge (abbreviations for ligands are also given in alphabetical order, i.e. CH 3 CN before MeCN before NCMe).
- Round brackets are used for polyatomic ligands (as well as abbreviations for ligands). Furthermore, the donor atom should be mentioned first if possible (for example, NCS and SCN are bond isomers ).
- The oxidation number can be specified as an exponent (Roman numeral) on the central atom (the positive sign is neglected). This information is optional.
- In polynuclear complexes, the type of binding of bridging ligands is denoted by the Greek letter μ . An index n indicates the number of linked central atoms. In the case of ligands with several donor atoms, the hapticity is indicated with the Greek letter η. Behind it is the number of donor atoms.
Complex names
In the systematic naming of complex salts, the cation is given first and then the anion , regardless of whether they are complex or not. The components of a coordination unit are named in the following order:
- Number of ligands : Multiple occurrences of ligands are indicated by using Greek numerals ( mono , di etc.) as a prefix. For ligands with more complex names or to avoid ambiguity (e.g. dithiosulphate ), their multiplicatives ( bis , tris etc.) are used. The part multiplied by this comes in brackets.
- Type of ligands : The various ligands are listed in alphabetical order, regardless of their number or charge. Anionic ligands are given the ending “o” in their anion names (e.g. chlorido ) and radicals have the ending “-yl” (e.g. nitrosyl ). The names of neutral or cationic ligands are not changed. Exceptions to this rule are the names of water ( aqua ), ammonia ( ammin ), CO ( carbonyl ) and NO ( nitrosyl ).
- Coordination unit: In the case of an anionic coordination unit, the central ion is given the Latin notation followed by the ending “at” (e.g. cuprate from Latin cuprum ). If the coordination unit is a cation or neutral, the unchanged German name is used.
- Charge of the central ion: The oxidation number of the central ion (atom) is indicated by a Roman number in round brackets and placed after the name of the coordination unit. A plus sign is not written, the Arabic number is used for zero.
The full name of the coordination unit is written in one word. Except for the names of the ligands aqua , ammin and nitrosyl , the names of all neutral ligands are put in brackets. The names of inorganic anionic ligands are put in round brackets if they already contain numerical prefixes or if this avoids ambiguity. In the name of complex salts, a hyphen is written between the names of the cation and the anion.
Building complexes
Central particles
Mainly transition metals come into consideration as central particles , which have correspondingly free d orbitals with which the ligands can connect. Complexes are just as common with lanthanides and actinides . The number of valence electrons has a decisive influence on the type, stability and structure of the complexes that are formed. Explanations for this can be found in corresponding theories, which will be discussed later. Examples of central particles are:
- cationic central ions: Cu 2+ , Mg 2+ , Fe 2+ , Fe 3+ , Ni 2+
- neutral central particles: Fe 0 , Cr 0 , Ni 0
Normally the metal particles in a complex have a positive oxidation number , but compounds with metal atoms in the oxidation state zero can also be produced by the reaction of metals or metal vapors with the corresponding ligands. An example is the reaction of nickel with carbon monoxide to form tetracarbonyl nickel ( Mond process ) or of iron to form pentacarbonyl iron . Such reactions can also be used to purify the corresponding metals ( chemical transport ). By reacting metals with ligands from the gas phase, for example, carbonyl , phosphine , olefin , aromatic and cyclopentadienyl complexes can be produced, although the formation of the latter is associated with a redox reaction.
Ligands
Complexes can consist of the same or different ligands. If a complex contains only ligands of the same type, it is called homoleptic , otherwise heteroleptic . Since in complexes the number of partners in a bond with the central particle is often independent of the oxidation state of the central particle, there is also the term “higher order compounds” from the 19th century for complexes.
Ligands can bring different numbers of electrons into the bond, for example Cl - and PPh 3 bring two electrons, η 5 -Cp and η 6 -C 6 H 6 six electrons, unbridged μ 1 -CO (see bridge (chemistry) ) two electrons, bridged μ 2 -CO one electron (see hapticity ). Ligands can not only bring in different numbers of electrons, but also coordinate with several points at the same central particle if they are of the appropriate size. The number of bonds possible here is called tenacity . The common simple ligands such as aqua (H 2 O), ammine ( NH 3 ), chlorido (Cl - ) or cyanido (CN - ) are all monodentate or monodentate , and bind e.g. E.g .: H 3 N-M.
Ligands that have several coordination sites for the same metal particle are called chelating ligands (Greek chelé , "crab claw "). The chelate complexes formed have a higher stability both thermodynamically and kinetically. The high thermodynamic stability is based on the increase in the entropy of the system, since the following reaction takes place in aqueous solution to form an octahedral complex with a bidentate ligand (ligand with two coordination sites), for example:
Here four free particles (on the left) become seven free particles (on the right). The kinetic stability is based on the fact that fewer particles have to meet in order to form the complex (according to the kinetic gas theory ) and all bonds of a ligand to the central particle have to be opened simultaneously during dissociation.
Common chelating ligands include bidentate ethylenediamine (s), tetradentate nitrilotriacetic acid (NTA) and hexadentate ethylenediaminetetraacetate (EDTA). The latter is used medically, for example, to bind toxic heavy metals such as lead or mercury in the body and then excrete them ( chelation therapy ).
Anionic ligands
Anionic ligands are formed from the name of the anion by adding the ending - o : -id becomes -ido , -it becomes -ito and -at becomes -ato . Some traditional names (such as chloro, cyano or oxo) were also allowed until the revision of the IUPAC nomenclature in 2005. As a result of the revision, these exceptions have now been eliminated and are no longer permitted.
- H - (hydrido)
- F - (fluorido) ; Cl - (chlorido) ; Br - (bromido) ; I - (iodido)
- O 2− (oxido) ; O 2 2− (peroxido ); OH - (hydroxido)
- Oxalate (ox)
- Acetylacetonate (acac)
- S 2− (thio) ; SO 4 2- (sulfato) ; S 2 O 3 2− (thiosulfato)
- SCN - (thiocyanato or isothiocyanato when coordinated via N)
- CN - (cyanido, isocyanido with coordination via N or cyano-C and cyano-N)
- NO 2 - (nitrito, nitro with coordination via N or nitrito-N and nitrito-O); NO 3 - (nitrato)
- Ethylenediamine tetraacetate EDTA 4− (ethylenediaminetetraacetato)
- Nitrilotriacetic acid (NTA 3− ) (nitrilotriacetato)
- Porphine / porphyrins (important in biochemistry )
Neutral ligands
- NH 3 (ammin)
- Ethylenediamine (s)
- H 2 O (aqua, obsolete aquo)
- CO (carbonyl)
- NO (nitrosyl)
Cationic ligands
- NO + (nitrosyl)
Organic ligands
- Cyclopentadienyl anion (Cp)
Spatial shape of complexes (molecular geometry)
Coordination number and coordination polyhedron
The coordination number (KZ) indicates with how many donor atoms of the ligand a central particle surrounds itself. Depending on the coordination number, the ligands arrange themselves in certain arrangements around the center, which often, but not always, agree with the predictions of the VSEPR model . If you think of the lines connecting the ligands, you get the coordination polyhedra, which are usually used to describe the structure of complexes. Coordination numbers from 2 to 9 are common, and numbers beyond this can only be achieved with particularly large central particles and chelating ligands. The most common, however, are the coordination numbers 4 and 6. According to the VSEPR model, the following polyhedra can be assumed:
- KZ 2 : a linear complex, e.g. B. [AuCl 2 ] - or angled complex z. B. [Au (tBuXanthPhos)] [AuBr 2 ]
- KZ 3 : a trigonal-planar or a trigonal-aplanar structure (the central particle is not exactly in the middle of the triangle, but slightly above)
- KZ 4 : a tetrahedron or a square-planar structure
- KZ 5 : a square-pyramidal or trigonal-bipyramidal structure; Both can be converted into one another by the Berry pseudorotation and are in equilibrium at the appropriate temperature
- KZ 6 : mostly an octahedron , sometimes a trigonal antiprism or (more rarely) a trigonal prism
- KZ 7 (very rare): a pentagonal bipyramid, a simply capped octahedron or a simply capped trigonal prism
- KZ 8 : a square antiprism, a trigonal dodecahedron , a doubly capped trigonal prism or, more rarely, a hex vein ( cube )
- KZ 9 : a triple capped trigonal prism, e.g. B. [ReH 9 ] 2− or capped square antiprism z. B. [Ln (thf) [N (CH 2 CH 2 OH) 3 ]] 3+
- KZ 12 : icosahedron or a cuboctahedron gives z. B. [Ce (NO 3 ) 6 ] 2- or [Zr (η 3 −BH 4 ) 4 ]
symmetry
Since the central particles and ligands of a stable complex, just like the ions, adopt geometrically ordered structures within crystal lattices , they are assigned to specific point groups . The labeling is usually based on the Schoenflies symbolism .
Isomerism
If there are several distinguishable compounds for one and the same empirical formula, one speaks (as with organic compounds) of isomerism . For complex compounds, the geometric isomerism , optical isomerism , ionization isomerism and bond isomerism are relevant.
Binding isomerism
Bond isomerism can occur when a ligand with different atoms can coordinate to the metal center. For example, the ligand SCN - can bond with the sulfur atom (thiocyanato) as well as with the nitrogen atom (isothiocyanato).
Geometric isomerism
Depending on the coordination number and composition of a complex compound, cis - trans isomerism or facial or meridional arrangement occurs.
cis - trans isomerism
The cis - trans isomerism occurs in square planar (coordination number KZ = 4) and octahedral (KZ = 6) complexes. The figure on the right shows the cis and trans isomers of an octahedrally coordinated compound of the general formula MA 4 B L , where L can represent both a further ligand of type B and a ligand of the third type C. For the designation, it is only important whether the two “special” ligands (twice B or once B and once C) are arranged towards one another ( cis ) or away from one another ( trans ). Accordingly, the four ligands of type A form a rocker ( cis ) or a square plane ( trans ).
If you mentally remove the two A ligands that are not on the equatorial plane in the sketch, you get a square planar complex with the general formula MA 2 BL. As with the octahedral complex, it is important whether B and L point towards each other ( cis ) or away from each other ( trans ). As soon as there are four different types of ligands in the square planar complex, the number of possible isomers increases to three.
fac and mer arrangement
The facial (fac) or the meridional (mer) arrangement occurs for octahedral complexes of the general formula MA 3 B 3 . Can be the ligands of the type A and clearly separate from type B by a plane containing the central particles M, each have three ligands of the fac-assembly in one direction, such as two opposite faces (engl. Faces ). If the ligands of the two types cannot be separated from each other, but can only be divided into orthogonal planes of the pure type A or B, one speaks of the mer arrangement , since there must always be three similar ligands on the meridian of the sphere, its surface includes all six ligands.
Optical isomerism
The prerequisite for optical isomerism is chirality , whereby a metal complex can also have a chiral metal center without a ligand molecule itself being chiral. Depending on the type of coordination, there are various requirements that must be met for optical isomerism to occur:
- tetrahedral (KZ = 4) metal complexes are chiral if four different ligands are bound to the metal center.
- Square planar (KZ = 4) metal complexes are chiral as soon as sterically demanding ligands interfere with one another and thus prevent rotation around a ligand-metal bond.
- Octahedral (KZ = 6) metal complexes often have chiral centers if they contain chelating ligands .
Formation of complexes
The classic complex formation reaction is an acid-base reaction according to the theory of Gilbert Newton Lewis . Here the central particle represents the Lewis acid (electron pair acceptor); the ligand is the Lewis base, i.e. a molecule or an ion which can provide at least one free electron pair (electron pair donor) to form a bond.
An example of a typical complex formation is the addition of water to cupric sulfate . The colorless salt reacts with the water to form a blue complex:
The Cu 2+ reacts as a Lewis acid and the water with its free electron pairs reacts as a Lewis base and a hexaqua complex is formed. This reaction is often used as evidence of water in chemistry classes at school because of its highly visible effect.
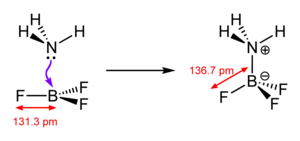
The type of chemical bond that results from the formation reaction is called coordinative bond (also - out of date - dative bond or donor-acceptor bond ), and thus from the other forms of chemical bond ( covalent bond , ionic bond , metal bond ) distinguished. This (controversial) distinction is justified by the fact that the binding pair of electrons originally mostly comes from the ligand and not (as in a covalent bond) one electron from each binding partner. In old textbooks, the bond is sometimes still indicated by an arrow in the direction of the acceptor, but these representations are out of date. Nowadays, a coordinative bond is drawn as a line in analogy to a covalent bond (see, for example, the sketch on the right), because although a complex is generally to be regarded as a Lewis acid-base adduct , the coordination can, as in In the area of homogeneous catalysis , also by oxidative addition , with part of the binding electrons being contributed by the metallic central atom, which can remain on this again in the course of a reductive elimination.
A typical example of a coordinative bond is the case shown in the adjacent sketch. Ammonia (NH 3 ) has a lone pair of electrons that are available for a coordinative bond to form the H 3 N-BF 3 molecule . Formally, the nitrogen atom transfers an electron to the boron atom, whereby the former (generally: the donor) receives a formal positive, the latter (generally: the acceptor) receives a formal negative charge. Note that these formal charges have nothing to do with the actual charge distribution: Since nitrogen has a much higher electronegativity than boron (3.0 versus 2.0), the bond to nitrogen is polarized (and the oxidation states remain unchanged).
In addition, there are complexes whose bonding relationships can only really adequately be described by more sophisticated concepts (such as molecular orbital theory ), such as metal clusters , sandwich complexes (e.g. bis (benzene) chromium , manganocene and ferrocene ) , but also the related half-sandwich compounds Olefin complexes (such as Zeise's salt ).
Binding relationships
The bond between central particles and ligands and the stability of complexes can be described in more detail using different models, which also enable statements to be made about properties such as color or magnetism.
VB theory
The earliest explanation was provided by the valence bond theory (VB theory). This assumes that occupied ligand orbitals overlap with unoccupied orbitals of the central particle and thus form a bond. To explain the spatial structure of complexes, it is assumed that hybrid orbitals are formed in metal. The VB theory explains the geometry and magnetic properties, but not, for example, the color. The 18-electron rule can also be used to estimate the stability of transition metal complexes in certain cases, although the scope of the rule is very limited.
Crystal and ligand field theory
The crystal field theory, which is based on pure electrostatic interactions between the ligands and the central particle and can also explain the color of the complexes, is a further development . The model is often mixed with the ligand field theory, which extends the crystal field theory and examines the influence of the point-like ligands on the energies of the d orbitals of the central metal. This approach is still very common today due to its ability to explain many properties despite its simplicity.
The energies of the d orbitals of a metal are initially degenerate (equal in energy). If a spherically symmetrical ligand field approaches, the energy of all d orbitals increases to the same extent. The ligand field theory now considers the spatial shape of these orbitals and the geometry of the ligand field in the form of point charges. If you look at an octahedral complex, for example, you will see that in an octahedral ligand field, due to the geometry, the d z 2 and d x 2 −y 2 orbitals are energetically less favorable, and the orbitals d xy , d yz and d xz in turn cheaper. The sum of these energy differences is called the ligand field stabilization energy (LFSE) and in an octahedral complex it is denoted by Δ O. The absolute value of this splitting can be determined experimentally by spectroscopy and is dependent on both the center and the ligands, but is generally given as 10 Dq. The influence of the centers and ligands on the splitting can be read off from the spectrochemical series .
The energy increase makes up 3/5 of the LFSE and the decrease accordingly 2/5. If all orbitals are occupied, the sum total is 0 Dq, since the energy center of the d orbitals must not change (dashed line in the figure). The energetically increased orbitals are referred to as e g orbitals ( e for twice “degenerate”) and the lowered orbitals as t 2 g orbitals ( t for “triple degenerate”). In a complex, these orbitals are now filled with electrons according to Hund's rule . In contrast to this, the formation of electron pairs instead of being occupied by unpaired electrons is also observed at high field splittings, provided the LFSE is higher than the spin pairing energy . Such complexes are called low-spin complexes, in contrast to the usual high-spin complexes.
Similar considerations can also be made for coordination polyhedra other than the octahedron. Ligand field theory can thus provide simple explanations, among other things, for the magnetic behavior of complexes by considering the pairing of electrons, or for the color, which can be explained with electron transitions between the orbitals. The geometric distortion caused by the Jahn-Teller effect can also be explained here using the electron configuration.
Spectrochemical series
The experimentally established spectrochemical series arranges ligands and metal particles according to the strength of the ligand field splitting they cause. The following sequence results for the ligands:
- I - <Br - <Cl - <F - <OH - <H 2 O <NC - <NH 3 <CN - <CO
In this series, the iodido ligand causes the smallest splitting and the carbonyl ligand the largest. This list corresponds in principle to the base strength according to the Lewis concept. You can sort the metal particles in a similar way:
- Mn 2+ <Ni 2+ <Co 2+ <Fe 2+ <V 2+ <Fe 3+ <Cr 3+ <V 3+ <Co 3+ <Mn 4+ <Mo 3+ <Rh 3+ <Pd 4 + <Ir 3+ <Re 4+ <Pt 4+
From this the rule of thumb becomes clear that higher ion charges also lead to higher splitting. The further to the right a particle is, the more likely it will be a low-spin configuration in a corresponding complex.
MO theory
However, the best results are provided by the molecular orbital theory, of which the ligand field theory is only a part. It treats both the central particle and the ligands quantum mechanically and is therefore the most precise, but also the most demanding.
stability
Several considerations can be taken into account in order to estimate or explain the stability of complex compounds.
HSAB concept
The principle of hard and soft acids and bases according to Ralph G. Pearson states that hard acids react preferentially with hard bases and soft acids correspondingly with soft bases. Particles with a high charge density are called hard and those with a low charge density that are easily polarized are called soft. This concept can also be applied to the stability of complex compounds.
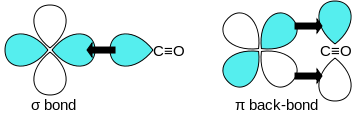
Tie back and forth
The MO theory provides further information about the stability of, for example, metal carbonyls . Accordingly, all ligands in complexes serve as σ donors, but strong ligands such as CO are also strong π acceptors. The σ-binding takes place via the HOMO of the CO, which overlaps with an empty d orbital of the metal. In addition, however, the LUMO of the CO can overlap with an occupied d orbital of the metal of suitable symmetry and thus brings about a π backbonding, which helps the complex to be particularly strong. In addition, this back-connection strengthens the σ-connection, which is why one speaks of a synergy here .
Application of the law of mass action
Equilibrium constants can be established for the quantitative description of the stability of complexes , since the Lewis acid-base reactions for complex formation are equilibrium reactions for which the law of mass action can be established. The overall reaction can be divided into individual steps (so-called elementary reactions), i. H. each for the addition of a ligand. The product of the equilibrium constants of the individual elementary reactions for complex formation then gives the equilibrium constant for the overall reaction.
The resulting constant is called the complex formation constant . This constant also indicates how stable the complex is or whether it tends to dissociate. Therefore, the complexation constant is also complex stability constant or complex association constant K A called. Their reciprocal value is called the complex dissociation constant K D , i.e. K D = K A −1 . The higher the complex formation constant K A , the more stable the complex, the smaller, the easier the dissociation.
Complex formation constants
According to Martell & Smith, 1982; Hyvönen & Aksela, 2010; Vasilev, et al. 1996; Vasilev, et al. 1998
| Complexing agents | abbreviation | Complex formation constant against Ca 2+ |
|---|---|---|
| Diethylenetriaminepentaacetic acid | DTPA | 10.8 |
| Ethylenediaminetetraacetic acid | EDTA | 10.7 |
| β-alanine diacetic acid | ADA | 5-7 |
| Methyl glycine diacetic acid | MGDA | 7th |
| Nitrilotriacetic acid | NTA | 6.4 |
| Nitrilotrimethylene phosphonate | NTMP | 5.75 |
| Tetrasodium iminodisuccinate | IDS | 5.2 |
| Tetrasodium N, N-bis (carboxylatomethyl) -L-glutamate | GLDA | 5.2 |
| Ethylenediamine disuccinic acid | EDDS | 4.6 |
Special complex compounds
Sandwich complexes
In sandwich complexes, the metal centers are enclosed by two planar and cyclic organic ligands like two halves of a bun, which is why this type of compound was given its name. The sandwich complexes include the metallocenes and the “piano chair complexes” . The most common ligand is the cyclopentadienyl anion (Cp), which has six π electrons and is therefore aromatic (see Hückel rule ). Corresponding complexes with benzene as a ligand are also possible, for example bis (benzene) chromium , or uranocene with cyclooctatetraenyl ligands.
The first sandwich complex to be synthesized was ferrocene in 1951 , but its possible structure puzzled for some time. Ernst Otto Fischer , Geoffrey Wilkinson and Robert B. Woodward finally clarified the correct structure independently of one another, for which the first two received the 1973 Nobel Prize in Chemistry. Ferrocene was discovered by chance while researching catalysts and was noticeable for the unusual stability of its orange-colored crystals. By fulfilling the 18-electron rule, it is more stable than similar compounds with other metals, such as cobaltocene or nickelocene .
In this type of compound, the organic ligands bind to the metal center with their π electrons. Since this does not necessarily have to happen with the entire ring, but other bonding states are also possible, the term hapticity is used to describe these relationships . The hapticity η in ferrocene is, for example, 5, since each ligand binds with five atoms. This is also reflected in the notation [Fe (η 5 -C 5 H 5 ) 2 ] for the complex.
Multinuclear complexes
Polynuclear complexes contain more than one central particle. They are linked via a bridging ligand such as oxygen (O 2− , OH - , H 2 O, OR) or chlorine. However, there are also complex compounds with (partly non-integer) metal-metal multiple bonds, e.g. B. [Tc 2 X 9 ] 3− , X = Cl, Br.
Macrocyclic Metal Complexes
Certain natural antibiotics of the cyclopeptide type (e.g. valinomycin ) are able to selectively bind and transport potassium ions. In 1967 Charles Pedersen first synthesized crown ethers , which belong to the type of macrocyclic polyethers and which are able to complex and transport especially alkali and alkaline earth ions . Starting from these macrocyclic polyethers, Jean-Marie Lehn's group in 1969 synthesized a macrobicyclic ligand (azo polyether ) for the first time, which was known as a cryptand . This also complexed alkali and alkaline earth ions in its cavity, the corresponding complexes were called cryptates . As a result, various cryptands were produced which have cavities of different sizes and are therefore adapted to the size of alkali or alkaline earth ions. The stability constants of the corresponding cryptates are relatively high, the complexes have a good selectivity for the ions and are therefore suitable for the selective separation of the ions from solutions. It was also possible to produce macrotricyclic cryptands and those with other heteroatoms. Many macrocyclic metal complexes also have biological significance . Further examples are complexes with phthalocyanine as a ligand, such as in the dye copper phthalocyanine .
Lattice-shaped metal complexes
Lattice-shaped metal complexes are supramolecular complex compounds made up of several metal atoms and coordinating chelate ligands that form a lattice-shaped structural motif. The structure is usually formed through thermodynamic self-organization . They have properties that make them interesting for information technology as future storage materials.
application
Biological importance
In biology complexes play an important role. These can be catalytically active proteins ( enzymes ) or catalytically inactive proteins. Numerous enzymes contain complexes in their active centers. This topic is one of the main areas of bioinorganic chemistry . In general, a complexing metal atom is present here which is not completely complexed by amino acid side chains as ligands. A ligand site acts as an active center for the conversion or temporary binding of the substrate. The most common complex centers are iron, copper, zinc, calcium, magnesium and manganese. But there are also more unusual elements such as vanadium. Calcium and zinc complexes in particular are of structural importance (e.g. zinc fingers in DNA sequence recognition).
The catalytically inactive proteins include z. B. Porphyrin complexes such as the heme in hemoglobin and in cytochromes , or chlorophyll (each chelate complex). Coordination compounds are therefore responsible for blood to appear red and a leaf of a plant to appear green.
technology
Complexes are used as catalysts in many chemical reactions . For example, in the already mentioned olefin metathesis , for which there was a Nobel Prize in 2005, carbene complexes with ruthenium or molybdenum are used (see Grubbs catalyst ). The Wilkinson catalyst is a square-planar rhodium (I) complex that is suitable for various applications such as the hydrogenation of olefins . It is also worth mentioning the industrial production of acetic acid from methanol and carbon monoxide with a rhodium catalyst in the Monsanto process .
Various complexing agents serve as food additives , as additives in the detergent and cleaning agent industry , in the electroplating and circuit board industry as well as in chemical analysis .
Phthalocyanine complexes are used in CDs as a storage medium.
In analog photography , the remaining, unexposed, hardly water-soluble silver bromide is removed from the layer with a fixing salt solution (ammonium or sodium thiosulphate ) after development : see fixing (photography) .
research
It is fundamentally a problem to fix short-lived and unstable molecules that occur as intermediate products in reactions. One method is fixation through complex formation. The fixed molecules have different chemical properties, however, in this way bonding and structural relationships can be investigated. Examples are complexes with carbenes , cyclobutadiene , diimines and carbene-analogous silylenes. After being released from the complexes, the molecules are again highly reactive. Metal complexes with chromium, nickel, iron and manganese are used. A metal carbonyl complex is often used as the starting complex . Examples: tricarbonyl cyclobutadiene iron, methoxyphenyl carbene pentacarbonylchromium, tetrachlorobis (tetramethylcyclobutadiene) nickel.
See also
- Organometallic chemistry
- Weakly coordinating ions
- Surface coordination chemistry
- List of ligand abbreviations
literature
- Lutz H. Gade: coordination chemistry . 1st edition, Wiley-VCH, Weinheim 1998, ISBN 978-3-527-29503-6 .
- Christoph Janiak: Complex / coordination chemistry , in: Erwin Riedel (Hrsg.): Moderne Anorganische Chemie . 3rd edition, de Gruyter, Berlin 2007, pp. 381-579, ISBN 978-3-11-019060-1 .
- AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , pp. 1315-1400.
- Henry Taube : Electron Transfer Between Metal Complexes - A Review (Nobel Lecture) , in: Angewandte Chemie 1984 , 96, pp. 315–326, doi : 10.1002 / anie.19840960504 .
Web links
- Learning units on complexes at Chemgapedia
- Learning units for pupils on complexes at Prof. Blum's educational server for chemistry
- Very extensive, interactively sortable list with complex formation constants
- Cambridge Structural Database
Individual evidence
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1316.
- ^ William H. Brock: Viewegs Geschichte der Chemie , Vieweg, Braunschweig 1997, p. 364.
- ↑ Lutz H. Gade: coordination chemistry . 1st edition, Wiley-VCH, Weinheim 1998, p. 6.
- ^ Alfred Werner: Contribution to the constitution of inorganic compounds . in: Zeitschrift für Anorganische Chemie 1893 , 3, pp. 267-330, doi : 10.1002 / zaac.18930030136 .
- ↑ Christoph Janiak: Complex / Coordination Chemistry , in: Erwin Riedel (Hrsg.): Moderne Anorganische Chemie . 3rd edition, de Gruyter, Berlin 2007.
- ^ A b c Nomenclature of Inorganic Chemistry , IUPAC Recommendations 2005 , RSC Publishing, Cambridge, UK.
- ^ IUPAC Red Book 2005, s. Section IR-9.2.2.3 for complexes (PDF file; 4.1 MB).
- ↑ Max Herberhold: Complex chemistry with naked metal atoms . In: Chemistry in Our Time . tape 10 , no. 4 , 1976, p. 120–129 , doi : 10.1002 / ciuz.19760100405 .
- ↑ Wolfgang Liebscher, Ekkehard Fluck: The systematic nomenclature of inorganic chemistry . Springer-Verlag, 1998, ISBN 978-3-540-63097-5 , pp. 127–150 ( limited preview in Google Book search).
- ^ Karl-Heinz Hellwich: More systematics: Nomenclature of Inorganic Chemistry. Edited by the International Union of Pure and Applied Chemistry. RSC Publishing, Cambridge / UK, 2005. XII + 366 pp., Hardcover, 49.95. ISBN 0-85404-438-8 . In: News from chemistry. 54, 2006, pp. 807-808, doi : 10.1002 / nadc.20060540725 .
- ↑ Entry on coordination . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.C01329 .
- ^ Erwin Riedel: Allgemeine und Anorganische Chemie , Walter de Gruyter Verlag (1999), Section 2.2.3, ISBN 3-11-016415-9 .
- ^ Charles E. Mortimer and Ulrich Müller: Chemistry. 10th edition, Thieme, Stuttgart 2010, p. 523.
- ↑ Dorota Kołodyńska: Chelating Agents of a New Generation as an Alternative to Conventional Chelators for Heavy Metal Ions Removal from Different Waste Waters P. 341, 345 doi : 10.5772 / 21180
- ^ Bernard Dietrich, Jean-Marie Lehn , Jean-Marie Sauvage: Kryptate: macrocyclic metal complexes . In: Chemistry in Our Time . tape 7 , no. 4 , 1973, p. 120–128 , doi : 10.1002 / ciuz.19730070405 .
- ↑ J.-M. Lehn et al., Angew. Chem., 2004, 116, pp. 3728-3747.
- ↑ Günter Schmid : The fixation of short-lived molecules through complex formation . In: Chemistry in Our Time . tape 8 , no. 1 , 1974, p. 26–30 , doi : 10.1002 / ciuz.19740080105 .


![{\ mathrm {[M (H_ {2} O) _ {6}] + 3 \ X \ longrightarrow [MX_ {3}] + 6 \ H_ {2} O}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/caaae75ddfe436b7995e9e8a56fd0d5a31e5a159)



![{\ mathrm {CuSO_ {4} +6 \ H_ {2} O \ longrightarrow [Cu (H_ {2} O) _ {6}] ^ {{2 +}} + SO_ {4} ^ {{2-} }}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/fec1a75e061a77a88df2e3e6becd39affc8cc852)


![{\ displaystyle {\ begin {aligned} \ mathrm {Ni ^ {2 +} + CN ^ {-}} & \ rightleftharpoons \ mathrm {[NiCN] ^ {+}} & \ quad K_ {1} & = \ mathrm {\ frac {[[Ni (CN)] ^ {+}]} {[Ni ^ {2 +}] \ cdot [CN ^ {-}]}} \\ [1em] \ mathrm {[Ni (CN) ] ^ {+} + CN ^ {-}} & \ rightleftharpoons \ mathrm {[Ni (CN) _ {2}]} & \ quad K_ {2} & = \ mathrm {\ frac {[[Ni (CN)) _ {2}]]} {[Ni (CN)] ^ {+} \ cdot [CN ^ {-}]}} \\ [1em] \ mathrm {[Ni (CN) _ {2}] + CN ^ {-}} & \ rightleftharpoons \ mathrm {[Ni (CN) _ {3}] ^ {-}} & \ quad K_ {3} & = \ mathrm {\ frac {[[Ni (CN) _ {3} ] ^ {-}]} {[Ni (CN) _ {2}] \ cdot [CN ^ {-}]}} \\ [1em] \ mathrm {[Ni (CN) _ {3}] ^ {- } + CN ^ {-}} & \ rightleftharpoons \ mathrm {[Ni (CN) _ {4}] ^ {2-}} & \ quad K_ {4} & = \ mathrm {\ frac {[[Ni (CN ) _ {4}] ^ {2 -}]} {[Ni (CN) _ {3} ^ {-}] \ cdot [CN ^ {-}]}} \\ [1em] \ mathrm {Ni ^ { 2 +} + 4CN ^ {-}} & \ rightleftharpoons \ mathrm {[Ni (CN) _ {4}] ^ {2-}} & \ quad K_ {A} & = K_ {1} * K_ {2} * K_ {3} * K_ {4} = \ mathrm {\ frac {[[Ni (CN) _ {4}] ^ {2 -}]} {[Ni ^ {2 +}] \ cdot [CN ^ { -}] ^ {4}}} = 1.0E + 29 \ end {aligned}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/672dae515b6e796be5c6c0a9e5332e200fd6fb14)