Sodium thiosulfate
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
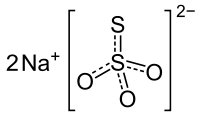
|
||||||||||
| General | ||||||||||
| Surname | Sodium thiosulfate | |||||||||
| other names |
Sodium hyposulfite (obsolete) |
|||||||||
| Molecular formula | Na 2 S 2 O 3 | |||||||||
| Brief description |
colorless, odorless, salty-bitter tasting crystals |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| Drug information | ||||||||||
| ATC code | ||||||||||
| properties | ||||||||||
| Molar mass | ||||||||||
| Physical state |
firmly |
|||||||||
| density |
1.67 g cm −3 (20 ° C) |
|||||||||
| Melting point |
45–50 ° C (pentahydrate) |
|||||||||
| boiling point |
Decomposition from 300 ° C |
|||||||||
| solubility |
good in water (701 g l −1 at 20 ° C) |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Sodium thiosulfate is the stable sodium salt of thiosulfuric acid, which is unstable in the free state .
Extraction and presentation
Sodium thiosulfate is made by stirring sulfur into boiling sodium sulfite solution:
properties
Sodium thiosulfate forms colorless crystals which crystallize with 5 mol of water of crystallization and are readily soluble in water; the liquid cools down considerably when it dissolves, since the enthalpy of hydration is smaller than the lattice energy and the missing amount of heat is withdrawn from the system. This so-called pentahydrate Na 2 S 2 O 3 · 5H 2 O is also known under the name of fixing salt because it is used for fixing during film development . Under the name of anti-chlorine , it is after the bleaching use of paper and textile fibers to remove excess chlorine.
The pentahydrate crystals have a melting point of 48.5 ° C, the melt can be supercooled and emits a large amount of heat of crystallization when solidified by a seed crystal. If acid is added to the aqueous sodium thiosulfate solution, sulfur separates out after a short time in the form of a yellowish cloudiness. The released, unstable thiosulfuric acid (H 2 S 2 O 3 ) breaks down quickly to sulfur and sulfur dioxide :
The water-insoluble silver halides ( AgCl , AgBr ) are dissolved by a fixing salt solution. The formation of the water-soluble sodium dithiosulfatoargentate (I) complex makes the developed film insensitive to light:
Sodium thiosulfate is a reducing agent and therefore reacts easily with the oxidizing agent potassium permanganate .
use
Sodium thiosulphate can be used as a fixing salt in analog photography , in mining for the extraction of silver chloride from silver ores and in electroplating for the production of gold and silver baths. In medicine , it is used as an antidote for cyanide poisoning , while less dangerous thiocyanate is formed.
Sodium thiosulfate is used as an antichlor to stop bleaching or disinfection processes with chlorine. The chlorine is reduced to chloride (or hydrochloric acid ) and hydrogen sulfate is formed :
In chemistry it is used to determine the iodine number , in iodometry thiosulfate is oxidized to tetrathionate :
- .
It is also used as a pentahydrate in so-called heat cushions . Bending a metal plate creates a crystallization nucleus which triggers the exothermic crystallization. The cushion is regenerated by heating it in boiling water, which melts (loosens) the crystals again.
In the context of studies, thiosulfate is used to prevent hearing loss, which can occur especially in children during chemotherapy containing cisplatin.
Individual evidence
- ↑ a b Entry on sodium thiosulfate. In: Römpp Online . Georg Thieme Verlag, accessed on November 11, 2014.
- ↑ a b c d e record with sodium thiosulfate in the GESTIS database of IFA , retrieved on December 20, 2019 (JavaScript required)
- ^ A b A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , p. 594.
- ^ AF Holleman , N. Wiberg : Inorganische Chemie . 103rd edition. Volume 1: Basics and main group elements. Walter de Gruyter, Berlin / Boston 2016, ISBN 978-3-11-049585-0 , p. 670 (reading sample: Part A - Basics of the chemistry of hydrogen. Google book search ).
- ^ Penelope R. Brock, Rudolf Maibach, Margaret Childs, Kaukab Rajput, Derek Roebuck: Sodium Thiosulfate for Protection from Cisplatin-Induced Hearing Loss . In: New England Journal of Medicine . tape 378 , no. 25 , June 21, 2018, p. 2376-2385 , doi : 10.1056 / nejmoa1801109 .



![{\ mathrm {2 \ Na_ {2} S_ {2} O_ {3} + AgCl \ rightarrow Na_ {3} [Ag (S_ {2} O_ {3}) _ {2}] + NaCl}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/bfb0ab7aad92851dce645ab6aa3774f8bb601209)

