Häme (group of substances)
Häme (from ancient Greek αἷμα haima , blood) are complex compounds with an iron ion as the central atom and a porphyrin molecule as a ligand . The best-known representative is Fe-Protoporphyrin IX, also called "Häm b " or simply "Häm". As a prosthetic group, heme can be found in several protein groups , including globins and cytochromes .
The group of substances is named for heme b , which is part of the red blood cells as an iron-containing dye . Together with the protein found in the erythrocytes , globin , it forms hemoglobin , which plays a central role in the body's absorption of oxygen. Heme other than heme b can be found in bacteria and plants.
Members
Haeme are iron-containing porphyrin complexes. The heme in the narrower sense initially differ in the functional groups that are bound to the porphyrin structure.
| Surname | Basic structure | R 3 | R 8 | R 18 | Occurrence |
|---|---|---|---|---|---|
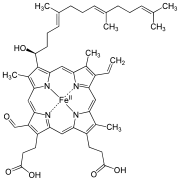
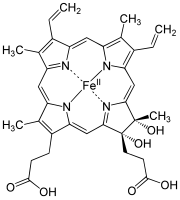
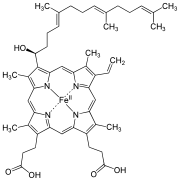
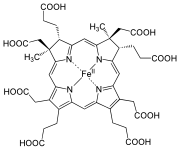
| Heme a |
|
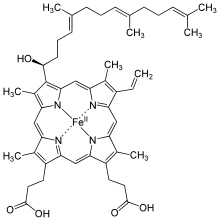
Hydroxyfarnesyl | −CH = CH 2 | −CH = O | Cytochrome c oxidase |
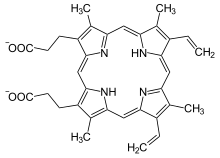
| Heme b | −CH = CH 2 | −CH = CH 2 | −CH 3 | Hemoglobin , myoglobin , catalase , succinate dehydrogenase , cytochrome c reductase , cyclooxygenase , cytochrome P450 | |
| Heme c | −CH (CH 3 ) SH | −CH (CH 3 ) SH | −CH 3 | Cytochrome c , cytochrome cd 1 | |
| Haem o | Hydroxyfarnesyl | −CH = CH 2 | −CH 3 | (Intermediate product) | |
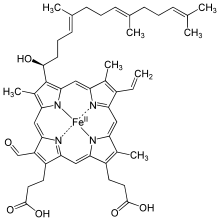
| Hamm s | –CHO | −CH = CH 2 | −CH 3 | Chlorocruorin |
While heme c is covalently bound to the thiol groups of cysteine residues in protein, heme a and b are in a non-covalent manner. Otherwise, heme b and c do not differ . By replacing certain amino acids with cysteine in the bound protein, b / c mixed forms can be generated that have different properties.
Other connections that belong to the heme are
- Heme d ,
- Häm d 1 , the prosthetic group of cytochrome cd 1 , a bacterial nitrite reductase ( EC 1.7.2.1 )
- Heme P460 , the prosthetic group of hydroxylamine oxidoreductase in Nitrosomonas europaea ( EC 1.7.3.4 )
- Sirohäm , the prosthetic group of ferredoxin nitrite reductase in plants, algae and cyanobacteria ( EC 1.7.7.1 ), as well as the bacterial and plant sulfite reductases ( EC 1.8.1.2 and EC 1.8.7.1 )
physiology
Hems are synthesized from the porphyrins in living things. The degradation pathways, on the other hand, lead via biliverdin and bilirubin to tetrapyrroles , the bile pigments , which cannot be further degraded and which are excreted.
biosynthesis
In the human body, the biosynthesis of all porphyrins starts from succinyl-CoA and the amino acid glycine . The first and at the same time rate-determining step is catalyzed by the enzyme δ-aminolevulinate synthase and leads to δ-aminolevulinate . Plants , algae , bacteria (with the exception of the alphaproteobacteria ) and archeal bacteria can also produce δ-aminolevulinate starting from glutamic acid, which is referred to as the C5 or Beale path. Every two δ-aminolevulinate molecules are condensed to form porphobilinogen, four of these molecules are then converted to hydroxymethylbilane . Further conversions take place step by step via uroporphyrinogen III and coproporphyrinogen III to protoporphyrinogen IX and protoporphyrin IX .
Heme b is produced by the incorporation of divalent iron into protoporphyrin IX with the help of the enzyme ferrochelatase .
Heme a, a component of cytochrome c oxidase, is produced from heme b in two steps . The mitochondrial protoheme IX farnesyltransferase (COX10) initially produces haem o , which is hydroxylated at the 18-position using another enzyme called COX15; Ferredoxin and the corresponding ferredoxin reductase are necessary as cofactors . The last step of conversion to the aldehyde is catalyzed by an unknown in the human enzyme in Bacillus subtilis as the gene product of CTAA - gene was identified.
Breakdown and excretion
The iron ion can oxidize, creating hematin , e.g. B. in methaemoglobin or in methaemalbumin .
The breakdown products of the heme are called bile pigments . The heme oxygenase converts the red heme still in the blood under demolition of the porphyrin ring and iron and elimination of carbon monoxide to green biliverdin to. Bile has a typical yellowish to greenish color, depending on the changing content of biliverdin and orange-red bilirubin ; the latter is produced by the biliverdin reductase by reduction from biliverdin. The bile pigments are mainly excreted with the urine (as yellow to orange Urochrome Sterkobilin , Urobilinogen and Urobilin ) or with the feces (as Coprochrome also Sterkobilin , Bilifuscin and Mesobilifuscin ).

Other bilirubin degradation products such as the colorless sterkobilinogen (which is converted by intestinal bacteria into the brown dipyrroles mesobilifuchsin and bilifuchsin) also contribute to normal stool color. Some of the bilirubin breakdown products are reabsorbed and excreted with the urine.
pathology
Each of the enzymes involved in biosynthesis and degradation can have a defect, which leads to a typical hereditary (rare) metabolic disorder . The defects occurring during porphyrin biosynthesis as well as the ferrochelatase deficiency are summarized under the name porphyria . Defects in the heme a synthesis, i.e. COX-10 and COX-15 deficiencies, on the other hand, lead to Leigh syndrome .
See also
- Haemines
- Haematins
- Chlorophyll , in which the iron ion of the heme has been replaced by a magnesium ion.
literature
- Ivano Bertini: Biological inorganic chemistry: structure and reactivity University Science Books, 2007, ISBN 1-891389-43-2 .
- PM Jordan: Highlights in haem biosynthesis . In: Curr. Opin. Struct. Biol. Vol. 4, 1994, pp. 902-911, PMID 7712294 .
- H. Panek, MR O'Brian: A whole genome view of prokaryotic haem biosynthesis. In: Microbiology. Vol. 148, 2002, pp. 2273-2282, PMID 12177321 .
- Berg, Tymoczko, Stryer: Biochemistry. 5th edition, Spektrum Akademischer Verlag GmbH, Heidelberg 2003, ISBN 3-8274-1303-6 .
Web links
- Heme and Porphyrin Metabolism (English).
Individual evidence
- ↑ Pallavicini A, Negrisolo E, Barbato R, et al. : The primary structure of globin and linker chains from the chlorocruorin of the polychaete Sabella spallanzanii . In: J. Biol. Chem. . 276, No. 28, July 2001, pp. 26384-90. doi : 10.1074 / jbc.M006939200 . PMID 11294828 .
- ↑ Bugiani M, Tiranti V, Farina L, Uziel G, Zeviani M: Novel mutations in COX15 in a long surviving Leigh syndrome patient with cytochrome c oxidase deficiency . In: J. Med. Genet. . 42, No. 5, May 2005, p. E28. doi : 10.1136 / jmg.2004.029926 . PMID 15863660 . PMC 1736058 (free full text).
- ↑ AL Sonenshein, James A. Hoch, Richard Losick : Bacillus subtilis and its closest relatives. ASM Press, 2002. ISBN 1-55581-205-8 , p. 174.
- ↑ Barros MH, Nobrega FG, Tzagoloff A: Mitochondrial ferredoxin is required for heme A synthesis in Saccharomyces cerevisiae . In: J. Biol. Chem. . 277, No. 12, March 2002, pp. 9997-10002. doi : 10.1074 / jbc.M112025200 . PMID 11788607 .
- ↑ Fontanesi F, Soto IC, Horn D, Barrientos A: Assembly of mitochondrial cytochrome c-oxidase, a complicated and highly regulated cellular process . In: Am. J. Physiol., Cell Physiol. . 291, No. 6, December 2006, pp. C1129-47. doi : 10.1152 / ajpcell.00233.2006 . PMID 16760263 .