Cytochrome c
| Cytochrome c | ||
|---|---|---|
|
||
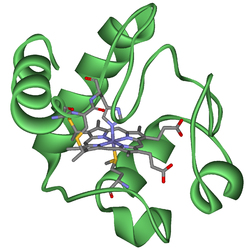
| Ribbon model of cytochrome c with the prosthetic group heme c as a rod model. | ||
| Properties of human protein | ||
| Mass / length primary structure | 104 amino acids | |
| Cofactor | Hamm | |
| Identifier | ||
| Gene name | CYCS | |
| External IDs | ||
| Occurrence | ||
| Parent taxon | Creature | |
Cytochrome c is a small protein from the cytochrome family that plays a crucial role in the mitochondria as an electron carrier during oxidative phosphorylation (energy generation). Orthologues of cytochrome c occur in all living things as mono- and multimers. In humans, mutations in cycs - Gen possible cause of cytochrome c deficiency and familial thrombocytopenia .
structure
The primary structure of human cytochrome c consists of 104 amino acids . The cytochrome c has a heme c as a prosthetic group , which is bound to two cysteine residues in the protein via two thioether bridges . The central iron (II) ion (Fe 2+ ) is octahedral complexed via coordinate bonds to the four nitrogen atoms (equatorial) of the pyrroles in the porphyrin and axially to a nitrogen atom of a histidine residue and to a sulfur atom of a Methionine residues in protein.
The heme group is also responsible for the red color of cytochrome c . A molten globule structure has also been described for cytochrome c .
function
The enzyme cytochrome c reductase (complex III of the respiratory chain ) oxidizes a molecule of coenzyme Q (CoQ) in the inner membrane of the mitochondria via the Q cycle and thereby reduces two molecules of cytochrome c that are located in the intermembrane space. The enzyme cytochrome c oxidase (complex IV of the respiratory chain) oxidizes four molecules of cytochrome c and reduces one molecule of oxygen to two molecules of water .
If the mitochondria are damaged, cytochrome c is released through the outer membrane into the cytosol , where it triggers programmed cell death ( apoptosis ) via a signal cascade .
Since cytochrome c occurs in practically all living beings and its primary structure differs between similar species only in a few amino acids, it is an important means for the taxonomic classification of living beings and an indication of the evolutionary development of species. Important groundwork for this was published by Walter M. Fitch .
See also
Enzyme complexes of the respiratory chain :
- Complex I, also NADH dehydrogenase
- Complex II, also known as succinate dehydrogenase
- Complex III, also cytochrome c reductase
- Complex IV, also cytochrome c oxidase
- Complex V, also known as ATP synthase
Individual evidence
- ↑ Homology group at OMA
- ↑ a b UniProt P99999
- ^ Walter M. Fitch : The molecular evolution of cytochrome c in eukaryotes. In: Journal of Molecular Evolution , Volume 8, No. 1, 1976, pp. 13-40, doi : 10.1007 / BF01738880
literature
- I. Bertini et al. (2006): Cytochrome c: occurrence and functions. In: Chemical Reviews . Vol. 106, pp. 90-115. PMID 16402772 .
