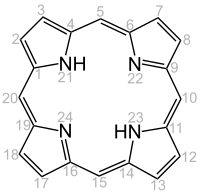
Porphyrins
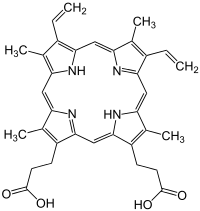
Porphyrins (from ancient Greek πορφυρά porphyrá , the purple dye ) are organic-chemical dyes that consist of four pyrrole rings ( tetrapyrrole ) that are cyclically linked by four methine groups . The simplest representative is porphine . Porphyrins or porphyrin-related compounds come e.g. B. also as chlorophyll and as heme in the heme-based proteins hemoglobin and the various cytochromes . Heme is a complex compound with protoporphyrin IX and an iron ion as the central atom instead of the two hydrogen atoms. An iron-free heme is e.g. B. Hematoporphyrin .
Importance of the porphyrins
Porphyrins, the structure of which was first described in detail by the chemist Hans Fischer , play a central role in the human metabolism. In addition to the transport of blood oxygen through the heme group bound in the red blood pigment, they occur in many enzymes, e.g. B. catalase (hydrogen peroxide detoxification) and the enzymes of the respiratory chain in the mitochondrion. The bacterially produced vitamin B 12 also contains a porphyrin-like component, namely corrin . Open-chain breakdown products of the red blood pigment in the body are responsible for the coloration of urine, stool and bile. Diseases related to porphyrins are e.g. B. hepatitis, in which the impaired breakdown of the heme leads to yellowing of the skin ( jaundice ), or the inherited heme build-up disorders ( porphyria ), in which precursor substances accumulate and lead to severe light intolerance, abdominal pain and nerve damage.
Some porphyrins can be used to determine drugs in the blood, e.g. B. by means of FPIA ( Fluorescence Polarization Immunoassay ), make impracticable or falsify.
| protein | Substance group | central metal | function |
|---|---|---|---|
| hemoglobin | Häme (group of substances) | Oxygen transport | |
| Photosystem | Chlorophylls | Light absorption | |
| Cytochrome c | Häme (group of substances) | Electron transport | |
| Cytochrome P450 | Häme (group of substances) | Oxygenases |
biosynthesis
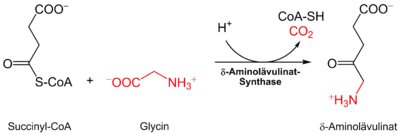
In the human body, the biosynthesis of all porphyrins starts from succinyl-CoA and the amino acid glycine . It takes place partly in the cytosol and partly in the mitochondrial intermembrane space. The reaction substrates and products therefore have to pass the mitochondrial membrane several times; the transport proteins responsible for this are still completely unknown.
The δ-aminolevulinate synthase catalyzes the connection of glycine and succinyl-CoA to α-amino-β-ketoadipate, which decarboxylates spontaneously to δ-aminolevulinate :
Plants , algae , bacteria (with the exception of the alphaproteobacteria ) and archeal bacteria can also produce δ-aminolevulinate starting from glutamic acid, which is referred to as the C5 or Beale path. The amino acid bound to a tRNA is reduced by glutamyl-tRNA reductase to glutamate-1-semialdehyde with NADPH consumption. This is finally converted into δ-aminolevulinate, which is catalyzed by glutamate-1-semialdehyde aminotransferase . However, it has not yet been proven that bacteria possess both the C5 pathway and the synthetic pathway described above via the δ-aminolevulinate synthase.
Four of these molecules are converted into hydroxymethylbilane by porphobilinogen deaminase, with the elimination of four ammonia molecules . Further conversions take place step by step via uroporphyrinogen III ( uroporphyrinogen III synthase ) and coproporphyrinogen III ( uroporphyrinogen decarboxylase ) to protoporphyrinogen IX ( coproporphyrinogen oxidase ) and protoporphyrin IX ( protoporphyrinogen oxidase ).
Breakdown and excretion
The hemooxygenase converts the red heme to green biliverdin by opening the porphyrin ring and splitting off iron and carbon monoxide . The biliverdin reductase reduces it to orange-red bilirubin . Bilirubin is secreted into the intestine via the bile and 75 to 80 percent of the bilirubin secreted is excreted with the feces , the rest is subject to the enterohepatic circulation .

Other degradation products of bilirubin such as the colorless sterkobilinogen (which is converted by intestinal bacteria to the brown dipyrroles mesobilifuchsin and bilifuchsin) and the orange-yellow sterkobilin contribute to the normal color of the stool. Bilirubin degradation products are partially reabsorbed and, as urobilinogen and urobilin, contribute to the yellow color of the urine .
Porphyrin-related molecules
 Porphyrin derivatives |
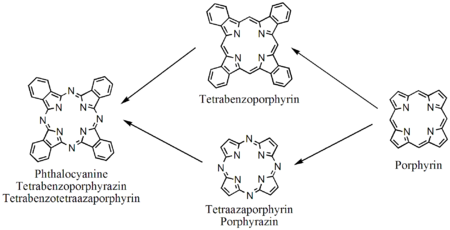 Tetraazoporphyrins, phthalocyanines |
Individual evidence
- ^ Otto Warburg : The Enzyme Problem and Biological Oxidations. In: Bulletin of the Johns Hopkins Hospital. Volume 46, 1930, pp. 341-358; also in: Albert Faulconer, Thomas Edward Keys: Otto Heinrich Warburg. In: Foundations of Anesthesiology. Charles C Thomas, Springfield (Illinois) 1965, pp. 1288-1302, here: p. 1301 ( The Chemical Constitution of the Enzyme ).
- ↑ EC 2.3.1.37 (δ-aminolevulinate synthase).
- ↑ EC 1.2.1.70 (glutamyl-tRNA reductase).
- ^ H. Panek, MR O'Brian (2002): A whole genome view of prokaryotic haem biosynthesis . In: Microbiology. 148, 2273-2282. PMID 12177321 .
- ↑ Beale, SI (1990): Biosynthesis of the tetrapyrrole pigment precursor, d-aminolevulinic acid, from glutamate. In: Plant Physiology. 93 (4), 1273-1279. PMID 16667613 ; PMC 1062668 (free full text, PDF).