Phthalocyanine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Phthalocyanine | |||||||||||||||||||||
| other names | ||||||||||||||||||||||
| Molecular formula | C 32 H 18 N 8 | |||||||||||||||||||||
| Brief description |
black to dark blue / purple colored crystals |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 514.54 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
> 300 ° C (decomposition) |
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Phthalocyanine (from phthalic acid ; and gr. Cyanos , κυανός, blue ) is the namesake of the phthalocyanines , a class of macrocyclic compounds with an alternating nitrogen-carbon ring structure. Structurally, they are similar to the related classes of organic dyes such as porphyrin and cyanines . Phthalocyanines are characterized by high chemical and thermal stability. Phthalocyanines - generally abbreviated as H 2 Pc - are resistant to concentrated sulfuric acid and can be sublimed at 500 ° C in a vacuum . In phthalocyanine, four benzopyrrole units are linked to one another via nitrogen (aza) bridges. The systematic name is tetrabenzotetraazaporphyrin .
history
The first appearance of an unknown blue by-product was reported in 1907. Today it is known that it was a metal-free phthalocyanine. The actual discovery of phthalocyanine as a dye also happened by chance when in 1928 at the Scottish Dyes Ltd. (ICI) in Grangemouth phthalimide should be produced from phthalic anhydride and ammonia in enamelled iron kettles. At one point where the enamel had flaked off to the point of iron , a dark blue substance had formed. In subsequent tests it was found that the blue color could not only be obtained with iron, but also by reacting phthalonitrile with other metals such as copper or nickel or their salts .
A year earlier, Henri de Diesbach and E. von der Weid had already reported on the synthesis and color brilliance of copper phthalocyanine in the journal Helvetica Chimica Acta , but without recognizing its economic potential as a pigment. The porphyrin- like structure of phthalocyanine postulated by Reginald Patrick Linstead in 1933 was confirmed by Robertson in 1935 by an X-ray structure analysis. It was therefore clear that the phthalocyanines resemble biologically relevant metal complexes such as the red blood pigment heme or the chlorophyll in plants.
The commercial production of copper phthalocyanine was started by ICI as early as 1934 . Bayer followed in 1936 and brought the substance onto the market as Heliogen Blue B. The previously used inorganic pigments ultramarine and Prussian blue were largely displaced in the following years. The range of metal phthalocyanine pigments was later expanded by replacing copper with cobalt or nickel, but also by chlorination ( phthalocyanine green ) or sulfonation (increased water solubility) of the base body.
Extraction and presentation
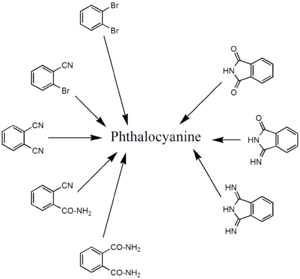
The phthalocyanine macrocycle is made up of four identical (corner) building blocks. Starting materials that correspond to these corners are therefore suitable as a synthesis strategy. These are usually derivatives of phthalic acid , such as phthalonitrile, phthalic anhydride, phthalimide or diiminoisoindole.
Conventional Techniques
On the one hand, the salts of various transition metals can be reacted with phthalonitrile and sodium methylate in a suitable solvent. On the other hand, elemental transition metals can be reacted with 1,3-diiminoisoindole in solution. In both cases, the corresponding metal phthalocyanine is obtained at 80-140 ° C., or H 2 Pc in the absence of metals or their salts. It is also possible to react various transition metal salts with urea and phthalic anhydride in an inert, high-boiling solvent using ammonium heptamolybdate as a catalyst and tetramethylurea as a promoter. The reaction solution requires a temperature of 120 to 250 ° C. The use of other phthalocyanine precursors is possible in this process, which is also used industrially. In the absence of metals or their salts, H 2 Pc is also formed here.
In the production of phthalocyanine pigments, if di- or trichlorobenzenes are used as solvents, chlorinated biphenyls can arise as undesirable by-products.
Microwaves
The synthesis of metal phthalocyanines by irradiation with microwaves is based on metal-free phthalocyanines which are reacted with the zinc, magnesium, cobalt or copper salts of acetic or hydrochloric acid. The product obtained here is the corresponding metal tetra- tert-butyl phthalocyanine. A reaction mixture free of any organic solvents is important for the reaction. Metal-phthalocyanine sandwich complexes (MPc 2 ) can only be produced in this way. Another possible application is the production of CuPc by reacting copper (I) chloride with urea and phthalic anhydride in the presence of a catalyst. The yield can be increased by using higher-energy microwaves.
Ultrasonic
Ultrasonic reactions can be carried out at room temperature. The time required varies from one minute to eight hours. On the one hand, copper phthalocyanine can be obtained by reacting phthalonitrile with copper (I) chloride in a suitable solvent. On the other hand, the reaction of copper (I) chloride with dichlorosilicone phthalocyanine monomers and a sodium chalcogen enables the synthesis of poly (phthalocyanato) siloxanes [Si (Pc) O] n .
Electrosynthesis
The electrosynthesis process is also a way of producing phthalocyanines at room temperature. Among other things, phthalonitrile is reacted on the anode with the respective metal salt in alcoholic solution on the cathode. Thus, using methanol, Cu, Ni, Co and the Mg-phthalocyanine complex can be prepared. The use of ethanol enables the synthesis of the Pb-phthalocyanine.
Irradiation (UV / VIS)
Irradiation of the reaction mixture consisting of phthalonitrile in an alcohol in the presence of sodium methoxide yields PcH 2 at room temperature .
Irradiation (laser)
For the production of copper phthalocyanine (CuPc), this method involves bombarding a copper target with a laser. The knocked out copper atoms can be incorporated into a thin film of 1,3-diiminoisoindole, whereby CuPc is obtained.
Other methods
In addition to the methods described briefly above, there are also possibilities to carry out syntheses at relatively low temperatures (<100 ° C). These thematically overlap with the electrosynthesis. It is also possible, inter alia, to produce phthalocyanine radicals by irradiation or electrochemical methods, which are stable under atmospheric oxygen. As a last method, for the sake of completeness, the preparation of phthalocyanine complexes using radioactive elements or radioactive isotopes of stable elements should be mentioned.
use
Phthalocyanines are used as dyes on optical data carriers ( CD-R ) and as pigments for plastics , lacquers and in the paper industry. They can also be used as photoconductors in laser printers or as electrode material in fuel cells . In chemical research , phthalocyanine is used as an easily produced model substance for the biologically important porphyrins . Phthalocyanine derivatives are also used in photodynamic therapy . In this process, phthalocyanines are accumulated in the tumor tissue and stimulated by light ( wavelength 600–800 nm ), which releases reactive singlet oxygen . Due to the associated secondary reactions, cell death occurs within a few hours due to necrosis and apoptosis , which ideally leads to total dissolution of the tumor after 4–6 weeks.
Phthalocyanine derivatives
- Aluminum phthalocyanine, CAS number: 14154-42-8
- Nickel phthalocyanine, CAS number: 14055-02-8
- Cobalt phthalocyanine , CAS number: 3317-67-7
- Iron phthalocyanine , CAS number: 132-16-1
- Zinc phthalocyanine , CAS number: 14320-04-8
- Copper phthalocyanine
- Polychloropper phthalocyanine
- Hexadecachlorphthalocyanine , CAS number: 28888-81-5
- Hexadecabromophthalocyanine , CAS number: 28746-04-5
- Manganese phthalocyanine, CAS number: 14325-24-7
Individual evidence
- ↑ Entry on CI 74100 in the CosIng database of the EU Commission, accessed on July 6, 2020.
- ↑ a b c d data sheet phthalocyanines at AlfaAesar, accessed on December 6, 2019 ( PDF )(JavaScript required) .
- ↑ a b data sheet 29H, 31H-Phthalocyanine, β-form, 98% from Sigma-Aldrich , accessed on December 6, 2019 ( PDF ).
- ^ The Phthalocyanines - Vols 1-4 Edited by CC Leznoff and ABP Lever, Wiley 1986-1993 .
- ^ Neil B. McKeown: Phthalocyanine Materials - Synthesis, Structure and Function , Cambridge University Press 1998 .
- ↑ The Porphyrin Handbook , Vols. 15-20; Karl Kadish, Kevin M. Smith, Roger Guilard (eds); Academic Press 2003 .
- ^ A. Braun, J. Tcherniac: "About the products of the action of acetic anhydride on phthalamide", in: Reports of the German Chemical Society , 1907 , 40 (2) , pp. 2709-2714; doi: 10.1002 / cber.190704002202 .
- ↑ H. de Diesbach, E. von der Weid: “Quelques sels complexes des o-dinitriles avec le cuivre et la pyridine”, in: Helvetica Chimica Acta , 1927 , 10 (1) , pp. 886-888; doi: 10.1002 / hlca.192701001110 .
- ↑ Peter Kredel: Production and use of PCB in the chemical industry. In: Hazardous substances - cleanliness. Air . 71, No. 1/2, 2011, ISSN 0949-8036 , pp. 7-9.
- ↑ BI Kharisov, U. Ortiz Méndez, JL Almaraz Garza, JR Almaguer Rodríguez: "Synthesis of non-substituted phthalocyanines by standard and non-standard techniques. Influence of solvent nature in phthalocyanine preparation at low temperature by UV treatment of the reaction system ", in: New J. Chem. , 2005 , 29 , pp. 686-692; doi: 10.1039 / b415712p .
- ↑ A. Hirth, U. Michelsen, D. Wöhrle: Photodynamische Tumortherapie , in: Chemistry in our time , 1999 , 33 (2) , pp. 84-94; doi: 10.1002 / ciuz.19990330204 .