Dyes
Dyes are colorants that, unlike pigments, are soluble in application media such as water or other solvents . They can be classified according to various criteria, for example according to their origin ( natural dyes / synthetic dyes), their use ( substrate ), their chemical structure ( chromophore ) or their field of application.
Some dyes can be converted into insoluble pigments by adding precipitants , see laking .
history
There is little knowledge about the use of dyes in ancient times, as they are relatively easily broken down by the action of light, air and microorganisms . Using modern analytical methods (for example HPLC ), however, the smallest traces of dyes can be detected. The blue, water-soluble dye indigotine was found on Egyptian textiles that are over 3000 years old . The cultivation of the indigo plant ( Indigofera tinctoria ) is already 2500 BC. In Egypt there is evidence of this and records of the dyeing process with indigo have come down to us from ancient times ( Papyrus Leidensis , Papyrus Holmiensis). In Europe this dye was obtained from woad . This approach became unprofitable in the 17th century when large quantities of indigo were imported from East India .

The use of real purple , obtained from the purple snail found on the coast of the eastern Mediterranean , can also be proven in antiquity. For red dyeing, the very expensive purple was partly replaced by the dye alizarin contained in madder ( Rubia tinctorum ), a dye that was known before the birth of Christ. Other dyes of natural origin that have been used since ancient times were henna , kermes , turmeric and saffron .
With the discovery of America natural dyes from wood (were Logwood , Redwood , Yellow Wood ) in the textile and leather dyeing, also for hair meaningful and paper color. The use of real carmine , obtained from cochineal shellfish ( Dactylopius coccus Costa), has also become popular in Europe .
The basis for the development of synthetic organic dyes was laid in the first half of the 19th century. Friedlieb Ferdinand Runge isolated and characterized in 1834 from coal tar , which is a by- product of the coking of coal , including aniline and phenol . The first synthetic dye was discovered by chance in 1832 by the German chemist Karl von Reichenbach and named Pittakall . The chemist August Wilhelm von Hofmann dealt with the chemistry of coal tar from 1843 and developed numerous new implementations and processes. Hofmann's student William Henry Perkin found in 1856 in oxidation tests the aniline Mauvein . This was the first synthetic dye made commercially. In the following years, the chemistry of tar paints , especially through the work of Hofmann and his students Perkin, Johann Peter Grieß , Carl Alexander von Martius (founder of Agfa ) and Georg Merck (founder of Merck & Co. in New York ), took a stormy development:
- 1856: First synthesis of the triphenylmethane dye fuchsin by the Polish chemist Jakub Natanson
- 1858: Development and patenting of a production of fuchsine by the French chemist François-Emmanuel Verguin . Almost at the same time, August Wilhelm Hofmann developed an alternative fuchsin synthesis.
- 1861: Invention of crystal violet by Charles Lauth - a dye from the group of cationic dyes , which is suitable for printing inks , inks and carbonless paper.
- 1862: Development of the diazotization reaction as an important intermediate step in the synthesis of azo dyes by Johann Peter Grieß. The azo dyes developed into the most important and largest group of synthetic dyes.
- 1863: Invention of Bismarck brown by Martius - the first commercially produced azo dye.
- 1869: First synthesis of a natural dye - alizarin - by BASF chemist Heinrich Caro in collaboration with Carl Graebe and Carl Liebermann .
- 1871: Development of the synthesis of the pH indicator phenolphthalein from phthalic anhydride and resorcinol by Adolf von Baeyer .
- 1877: Synthesis of malachite green by Otto Fischer .
- 1878: First full synthesis of indigo by Adolf von Baeyer. An alternative production process was developed by Karl Heumann in 1890 , which, after further improvements, led to the industrial production of the dye from 1897. The cost-effective industrial indigo production, which was achieved through further process optimization - in particular by the chemist Johannes Pfleger - led in a few years to a drastic reduction in the market share of naturally obtained indigo.
The successful large-scale production and marketing of the new synthetic dyes was important for this development. So Perkin secured his invention with a patent and founded a chemical factory in which mauvein was produced as a dye for dyeing silk and cotton as early as 1857 . In Germany, the later Hoechst AG (on January 2, 1863 as Theerfarbenfabrik Meister, Lucius & Co. ) and Bayer AG (on August 1, 1863 as Friedr. Bayer et comp. ) And, two years later, BASF (on 6. April 1865 as Baden aniline and soda factory ).
A further development in dye chemistry in the 20th century was the introduction of lightfast vat dyes based on anthraquinone . The first representative in 1901 was the dye indanthrene blue developed at BASF , from which the extensive indanthrene range developed. In the 1920s, the invention of disperse dyes made it possible to dye hydrophobic synthetic fibers , such as acetate silk or later polyester fibers . With the development of phthalocyanines , especially copper phthalocyanine , a new metal-containing chromophore was produced by ICI from 1935 , which was initially used as a pigment. By introducing solubilizing sulfonic acid groups, this chromophore could also be used as a dye. In 1951 the first reactive dyes for dyeing wool were introduced, and from 1956 reactive dyes for cotton dyeing. In the following decades, the focus of textile dye development was on improving the application properties. For example through the development of bifunctional reactive dyes or reactive dyes with new reactive groups.
In addition to textile applications, functional dyes have increasingly come into focus over the years. This term was coined in 1993 for dyes whose specific application is not based on their aesthetic color properties. Functional dyes are used in medicine, pharmacy, photovoltaics, data storage and the printing industry, among others.
Chemical-physical basics
For humans, the light spectrum is visible in the wavelength range between 380 and 790 nm. When white light hits a body, the light spectrum is partly reflected and partly absorbed. For example, if short-wave components of the light color ( violet to blue , 420-480 nm) are absorbed, the remitted radiation mainly contains long-wave components (up to 780 nm) and the color impression, the so-called body color , is yellow to red .
Dyes also absorb and reflect part of the visible white light and the mixture of the complementary colors of the absorbed light is perceived by the eye.


The coloring properties of a chemical compound result from its chemical structure. While molecules with σ bonds absorb electromagnetic energy in the X-ray and UV range , molecules with electrons in π bonds (unsaturated bonds) are already excited by electromagnetic radiation with lower energy. If there are several conjugated unsaturated bonds in the molecule, the π-electrons are delocalized and as the degree of conjugation increases, the energetic distance between the ground state and the excited energy state of the molecule decreases . The absorption maximum shifts towards longer wavelengths and into the visible range of the spectrum. (→ bathochromic effect )
Such molecular structures are called chromophores according to the dye theory of Otto Nikolaus Witt . Functional groups in the molecule that act as electron donors or as electron acceptors influence the mesomerism in the molecule by increasing or decreasing the electron density of the chromophoric group. They are also known as auxochromes or antiauxochromes.
Auxochromic groups are, for example, the hydroxyl , ether , amino and amido groups , and antiauxochromic groups are the carbonyl , nitro , carboxyl and sulfo groups .
Classification of dyes
The most common classification of the various dyes is based on their chemical structure or according to their dyeing application process.
Classification according to chemical structures
Anthraquinone dyes
The basic structure of this group of dyes is anthraquinone . By varying the substituents, almost all hues from yellow to red and blue to green can be achieved, with the red and blue anthraquinone dyes being particularly important. The quinoid system can be converted into the corresponding water-soluble hydroquinone by reduction , so that the anthraquinone dyes can be used as vat dyes . With suitable substituents, anthraquinone dyes can be used as disperse dyes for dyeing synthetic fibers. Water-soluble anthraquinone dyes with sulfonic acid groups are used as acid or reactive dyes .
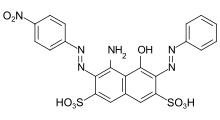
Azo dyes
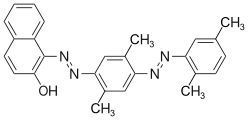
The azo dyes contain an azo group substituted by aryl or alkenyl radicals as their basic structure . Azo dyes with several azo groups are referred to as bisazo (also disazo), trisazo, tetrakisazo, polyazo dyes. Aryl substituents are generally benzene or naphthalene derivatives, but also heterocyclic aromatics, such as pyrazoles or pyridones . Enolizable aliphatic groups, for example substituted anilides of acetoacetic acid , are used as alkenyl substituents .
The dye synthesis takes place by diazotization of aromatic amines and subsequent azo coupling of the diazonium salt to electron-rich aromatics or β-dicarbonyl compounds. The azo dyes are by far the most important and extensive group of dyes and are represented in almost all application engineering categories (→ classification according to application engineering processes ). No naturally occurring azo dyes are known. With the exception of turquoise or a brilliant green , almost all color tones can be achieved with azo dyes. The azo group is sensitive to reducing agents - it is split and the dye thereby discolored.
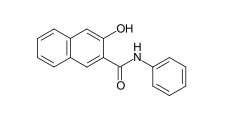
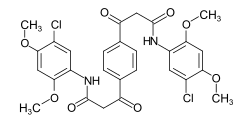
Some examples of different azo dye types (mono- and bisazo dyes / benzene, naphthalene residues / pyridone, acetoacetanilide coupling component / metal complex dye):
Dioxazine dyes
Dioxazine dyes - also known as triphendioxazine dyes - contain triphendioxazine as a basic structure. The strong, brilliant dyes have good fastness properties and thus combine the advantages of the azo dyes and the anthraquinone dyes. Dioxazine dyes are commercially available as direct and reactive dyes.
Indigoid dyes
The indigoid dyes belong to the carbonyl dyes and are used as vat dyes. The most important representative is indigo, which was obtained as a natural dye from plants in antiquity and which is still industrially produced in large quantities and used in particular for dyeing blue jeans .
Another natural dye is ancient purple ( CI Natural Violet 1 / Dibromoindigo ).
Metal complex dyes
Metal complex dyes are coordination compounds of a metal ion with one or more dye ligands that have electron donor groups. Copper and chromium compounds predominate , but cobalt , nickel and iron complexes are also used as dyes to a lesser extent . The ligands are often azo dyes , azomethine dyes , formazans or phthalocyanines . The metal complex dyes are distinguished by very good fastness properties.
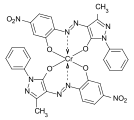
Formazan dyes
The formazan dyes are structurally related to the azo dyes. The basic structure is 1,3,5 ‑ triphenylformazan . They form chelate complexes with transition metals such as copper , nickel or cobalt . Depending on the other substituents, the non-complexed formazans are orange to deep red, the metal complex formazans purple, blue to green. The synthesis is carried out by coupling of diazonium salts of hydrazones .
Blue tetradentate copper chelate complexes of various formazans, which are used in particular as reactive dyes for cotton, are of commercial importance:
Phthalocyanine dyes
The phthalocyanine dyes are copper - or nickel - metal complexes with the basic structure of the phthalocyanine . They are structurally related to the porphyrins , with which they share the aza [18] annulene element. By introducing water-soluble substituents - mainly via sulfochlorination - turquoise to brilliant green dyes are accessible. The phthalocyanine dyes are characterized by excellent lightfastness.
Methine dyes
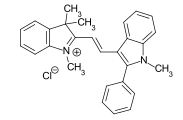
The methine or polymethine have as a chromophoric system conjugated double bonds, with two end groups as electron acceptor A and electron donor D function. The end groups, which in most cases contain nitrogen or oxygen atoms, can be part of a heterocycle and the double bonds part of an aromatic system. If one or more methine groups are replaced by nitrogen atoms, they are called aza-analogous methine dyes. This results in different subclasses:
- Cyanine dyes in which the conjugated double bonds are flanked by a tertiary amino group and a quaternary ammonium group . If two methine groups are replaced by nitrogen atoms and one terminal group is part of a heterocycle, while the second terminal group is open-chain, the important diazahemicyanine dyes are obtained. Example: CI Basic Red 22 .
- Styryl dyes : by inserting a phenyl ring into the polyene structure, they have a styrene structural element . Example: CI Disperse Yellow 31 .
- Triarylmethine dyes, also referred to as triarylmethane dyes in the older literature , since they are derived from triphenylmethane in which at least two of the aromatic rings have electron-donating substituents. Example: CI Basic Green 4 (malachite green).
Nitro and nitroso dyes
In the case of nitro dyes, there is a nitro group on an aromatic ring in the ortho position to an electron donor - either a hydroxy (-OH) or an amino group (-NH 2 ). The oldest representative of this class of dyes is picric acid (2,4,6-trinitrophenol). However, the hydroxynitro dyes are no longer of commercial importance. It is a relatively small but old class of dyes, the representatives of which are characterized by a high level of lightfastness and ease of manufacture. The nitro dyes are yellow to brown in color. Since they are relatively small molecules, an important area of application as disperse dyes is the dyeing of polyester fibers. They are also used as acid and pigment dyes.
The rare nitroso dyes are aromatic compounds with a nitroso group . Nitroso dyes with a hydroxyl group in the ortho position to the nitroso group are used exclusively as metal complexes. A typical representative is Naphtol Green B (CI Acid Green 1).
Sulfur dyes
Sulfur dyes (sulfine dyes) are water-insoluble, macromolecular dyes that have disulfide or oligosulfide bonds between aromatic radicals. They are obtained by melting benzene , naphthalene or anthracene derivatives with sulfur or sodium polysulphides and are of a non-uniform constitution . They are particularly suitable for dyeing cotton and, like vat dyes, are reduced to the water-soluble form with caustic soda and dithionites or sodium sulfide ( leuco compound ) and, after being attached to the fiber, fixed insoluble on the fiber by oxidation . For toxicological and ecological reasons, oxidation with dichromate is now largely avoided, and sulfur dyes that are low in sulfide and sulfide-free reducing agents are being used more and more. Due to the low production costs, the sulfur dyes still play an important role in terms of quantity. Sulfur dyes are particularly washable and lightfast, the colors are usually muted.
Classification according to application technology
While the color shade of a dye is essentially determined by the chromophore, the properties of the dyes can be varied by incorporating suitable chemical groups in such a way that different substrate types can be colored. This results in a classification of the different dyes according to the dyeing process. This classification is also followed by the Color Index , an important standard work in the field of dye chemistry. From the color index (CI) the dyer can recognize which dye class, which color and which substance it is. The CI contains more than 10,000 dyes - more than 50% of them are azo dyes.
Mordant dyes
The name is derived from the dyeing process in which suitable acid dyes are applied to the stained material - mainly wool and silk. In order to dye with stain dyes, the fibers to be dyed are first treated with chromium (III), iron (III) or aluminum salts and stained. During the subsequent treatment with steam, metal hydroxides are formed on the fiber. When coloring, these hydroxides react with the (mostly special) acid dye to form a metal complex dye . The process on the fiber corresponds to varnishing .
If chromium salts are used, one speaks of chromizing dyes. Depending on the type of staining dye , the chromium salt - usually chromates or dichromates - can be added before, during or after dyeing. A distinction is made accordingly between pre, post and single bath chromating processes. The chromating dyes are particularly notable for their very good wet fastness. However, the heavy metal pollution of the fibers and the dyeing waste water is a disadvantage and ecologically critical.
The stain dyes are designated as CI Mordant Dyes according to the Color Index .
Examples:
Direct dyes
Due to their high substantivity, direct dyes (or nouns dyes) are absorbed directly from aqueous solution onto the fiber. They are particularly suitable for use on cellulose . These dyes are bound to the fiber through physical interactions ( van der Waals bonds ). Most representatives come from the group of azo dyes, preferably polyazo dyes.
The direct dyes are referred to as CI Direct Dyes according to the Color Index .
Examples:
Disperse dyes
The almost water-insoluble disperse dyes are preferably used for dyeing hydrophobic polyester and acetate fibers . They are ground very finely together with dispersants , which means that the molecularly dissolved dye components can diffuse into the fiber during the dyeing process, where they form a solid solution, resulting in dyeings which are washable and lightfast.
The vast majority of disperse dyes belong to the azo dyes. Disperse dyes are a very important group of dyes, especially due to the mechanically high quality polyester fibers. The total amount traded in 1999 for Western Europe had a sales value of 98 million euros.
The disperse dyes are referred to as CI Disperse Dyes according to the Color Index .
Examples:
Developing or coupling dyes
In the case of developing dyes, a practically water-insoluble dye is formed directly on the fiber by the reaction of a water-soluble coupling component ( CI Azoic Coupling Component ) with a water-soluble diazo component ( CI Azoic Diazo Component ). This class of dyes is mainly used to dye cellulose fibers, the dyeings being characterized by very good wet fastness. The most important coupling component in developing dyes is CI Coupling Component 2 (Naphthol AS) .
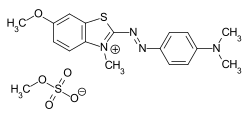
Cationic dyes
Cationic dyes are positively charged compounds which, when combined with polyacrylonitrile fibers (PAN) and anionically modified polyester , produce brilliant and lightfast colors. They form ionic bonds with negatively charged groups on the fiber. Different chromophores can be used for cationic dyes, with the positive charge in the methine dyes being delocalized in contrast to other chromophoric systems.
Even if the cationic dyes are named CI Basic Dyes according to the Color Index , the term basic dyes for this group of dyes is no longer used in the more recent literature.
Vat dyes
The vat dyes include water-insoluble pigments which are converted into their soluble dihydro- or leuco form by reduction ( vatting ) in an alkaline solution for coloring . The anion shows a sufficient affinity for cotton or viscose fibers so that the vat dye can be absorbed. Here it is converted back into the insoluble state by subsequent oxidation . This can be done either by atmospheric oxygen or treatment with oxidizing agents. The dye is quasi molecularly fixed to the fiber, this "precipitation in the fiber" causes the high washing and lightfastness. The water-insoluble sulfur dyes also behave like the vat dyes.
The most important vat dye is indigo . The indanthrene dyes are also important.
The vat dyes are designated as CI Vat Dyes according to the Color Index .
Examples:
Food coloring
The food colors are used as food additives to compensate for processing-related color changes or to satisfy the color expectations of consumers. Both dyes of natural origin and synthetically produced dyes are used. The use of dyes as food coloring is strictly regulated by law - in the EU by EC Regulation No. 1333/2008 of December 16, 2008 on food additives. Only approved food additives with an E number may be placed on the market. These additives must be identified on the product.
The food dyes are designated as CI Food Dyes according to the Color Index .
Solvent dyes
The solvent dyes , named according to the Color Index Solvent Dyes , are water-insoluble dyes that are soluble in various organic solvents such as alcohols, esters or hydrocarbons. As a rule, the structures of the solvent dyes do not contain any sulfonic acid or carboxyl groups. Cationic dyes with an intramolecular sulfonate or carboxylate group as a counteranion are excluded. Representatives of the solvent dyes can be found in various chemical dye classes, from the azo dyes, through anthraquinone dyes, metal complex dyes to the phthalocyanines. Solvent dyes are used as a component of paints (example: dyes for Zapon varnishes ), for coloring mineral oil products ( Sudan dyes ), wax , inks and various transparent plastics.
The solvent dyes are referred to as CI Solvent Dyes according to the Color Index .
Examples:
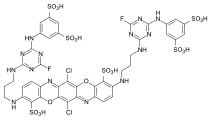
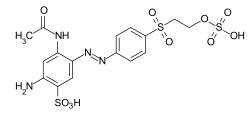
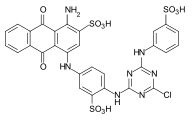
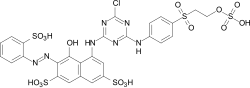
Reactive dyes
During the dyeing process, reactive dyes form a covalent bond with the functional groups of the fiber , which results in wetfast dyeings. They are the largest group of dyes for dyeing cellulose, but they can also be used to dye wool and polyamide in deep shades.
From a chemical point of view, reactive dyes consist of two parts - a chromophore and one or more reactive groups, also known as reactive anchors. Two different reactive anchor systems are important:
- Heterocyclic compounds, for example halogen-substituted triazine or pyrimidine derivatives . During the dyeing process, these react with the hydroxyl groups of the fibers, splitting off hydrogen halide and forming a stable covalent ether bond :
- The so-called vinyl sulfone group , which reacts during the dyeing process with the nucleophilic groups of the fiber in the sense of a Michael addition . In this case, too, a stable ether bond is formed. In the case of many vinyl sulfone dyes , the vinyl sulfone group is in a protected form as sulfuric acid half-ester. The vinyl sulfone group is only formed by the elimination of sulfuric acid under the alkaline dyeing conditions .
The two reactive anchor types can also be present in parallel in a reactive dye.
As a chromophore, the azo dyes are by far the most common of the reactive dyes. However, other chromophoric systems such as anthraquinone, formazan and phthalocyanine dyes also play an important role.
The reactive dyes are referred to as CI Reactive Dyes according to the Color Index .
Examples:
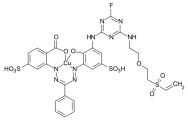
Acid dyes
Acid dyes have hydrophilic , anionic substituents - usually these are sulfonic acid groups . Most representatives of this class of dyes belong to the azo dyes, but there are also acid dyes with other chromophores. They are mainly used to dye wool, silk and polyamide, the dyeing process taking place in the pH range 2-6. When using small dye molecules, uniform colorations are obtained in which the dye molecules mainly form salt-like bonds with the ammonium groups of the fiber. The washout behavior ( wet fastness ) is rather moderate with these products. As the molecular size increases, the binding of the dye increases through adsorption forces between the hydrophobic part of the dye molecule and the fiber. This improves the wet fastness, but in many cases at the expense of the evenness (levelness) of the dyeing.
The acid dyes are referred to as CI Acid Dyes according to the Color Index .
Examples:
CI Acid Blue 3 ( patent blue V )
Functional dyes
While conventional dyes are used, for example, to change the appearance of textiles, leather and paper, functional dyes generally have no aesthetic purpose. Typical areas of application are indicator dyes or voltage-dependent dyes
Special dyes can
- Absorb light at a certain wavelength and convert the absorbed light into heat (e.g. in chemical and biochemical analysis),
- re-emit the light at a different wavelength (as phosphorescent biomarkers or inks, fluorescence in dye lasers, chemiluminescence to break or re-establish chemical bonds in biochemistry),
- change the direction of polarization of the light (as with frequency doubling or as an optical switch),
- cause electrical phenomena (used in laser printers),
- enable photochemical processes.
From an economic point of view, the use of functional dyes is particularly important for the production of CDs and DVDs. The dye molecules are contained in the polycarbonate of a CD or DVD. Dye molecules absorb light energy through the laser beam of the burner and convert it into heat. The plastic, the polycarbonate, melts at this point due to the absorption of heat. The surface has changed slightly, the changed surface structure is perceived during the reading process.
Industrial dye production
In addition to chemical reactions, industrial dye production includes various physical processes, such as salting out , filtration , reverse osmosis (RO) , drying and grinding.
Before or after drying, the commercial dyes are standardized to a certain color strength by adding colorless, indifferent adjusting agents such as sodium sulfate, sodium chloride or dextrin, in order to compensate for the fluctuations in different production lots. Targeted addition of other dyes (shading dyes) can guarantee the color consistency of the commercial product. The storage stability of the products can in some cases be increased by adding buffer substances such as mono- and disodium phosphate .
In particular with poorly water-soluble dyes, grinding has a major influence on the dyeing process (color tone, color strength ). In the 1960s and 1970s, in addition to dye powders, increasingly concentrated aqueous dye solutions were marketed. With the introduction of liquid brands, such as the development of dye granulates, automatic dye dosing in the dye works has been made easier and dust pollution has been reduced.
Before 1980, industrial dye production was very well represented in Western Europe, North America and Japan. Increased environmental protection costs and increasing import pressure from Asia by many non-traditional dye manufacturers, particularly from China, India, Korea and Thailand, led to considerable restructuring among traditional dye manufacturers. Acquisitions and joint ventures led to a concentration of the dyes business, as a result of which many production facilities were closed.
Large traditional dye manufacturers are Huntsman (formerly Ciba ), DyStar (emerged from the dye divisions of Hoechst AG , Bayer AG and BASF ) and Archroma (emerged from Clariant ). These traditional dye manufacturers now produce mostly in Asia.
See also
- List of dyes , sorted by color
- Dyeing in antiquity
literature
- Heinrich Zollinger: Color Chemistry: Syntheses, Properties, and Applications of Organic Dyes and Pigments . 3. Edition. WILEY-VCH Verlag, Weinheim 2003, ISBN 3-906390-23-3 ( limited preview in the Google book search).
- Klaus Hunger (Ed.): Industrial Dyes: Chemistry, Properties, Applications . WILEY-VCH Verlag, Weinheim 2003, ISBN 3-662-01950-7 ( limited preview in the Google book search).
- Jürgen Fabian, Horst Hartmann: Light Absorption of Organic Colorants. Theoretical Treatment and Empirical Rules . Springer, Berlin 1980, ISBN 3-642-67589-1 ( limited preview in the Google book search).
- Martin Klessinger: Constitution and light absorption of organic dyes . In: Chemistry in Our Time . tape 12 , 1978, p. 1-11 .
- Guido Ebner, Dieter Schelz: Textile dyeing and dyes . Springer-Verlag, Berlin 1989, ISBN 3-642-70172-8 ( limited preview in the Google book search - reprint 2011).
- Wilfried Kratzert, Rasmus Peichert: Dyes . Quelle & Meyer, Heidelberg 1981, ISBN 3-494-01021-8 .
- Helmut Schweppe: Handbook of natural dyes. Occurrence - use - evidence . ecomed, Landsberg / Lech 1993, ISBN 3-609-65130-X .
- Lutz Roth, Kurt Kormann, Helmut Schweppe: Dye plants - plant colors. Botany - dyeing methods - analysis - Turkish carpets and their motifs . ecomed, Landsberg / Lech 1992, ISBN 3-609-65490-2 .
- Sabine Struckmeier: Natural dyes: colors with history . In: Chemistry in Our Time . tape 37 , no. 6 , 2003, p. 402-409 , doi : 10.1002 / ciuz.200300275 .
- Herbert Vogler: Dyeing has been going on for thousands of years - an overview of ancient dyeing . In: textile finishing . tape 21 , no. 6 , 1986, pp. 229-235 .
- Herbert Vogler: 150 years of the dye industry. Part I . In: textile finishing . tape 42 , no. 11/12 , 2007, pp. 11-14 ( tib.eu ).
- Herbert Vogler: 150 years of the dye industry. Part II . In: textile finishing . tape 43 , no. 1/2 , 2008, p. 10-14 ( tib.eu ).
- Anthony Travis: The Rainbow Makers: The Origin of the Synthetic Dyestuff Industry in Western Europe . Lehigh Univ. Press, Bethlehem / London / Toronto 1993, ISBN 0-934223-18-1 .
Web links
- Thomas Seilnacht: Lexicon of pigments and dyes on seilnacht.com, accessed on January 7, 2017.
- Lexicon of Dye Plants on digitalefolien.de, accessed on January 7, 2017.
- J. Scherkenbeck: Dyes ( Memento from January 8, 2017 in the Internet Archive ) (PDF; 2.16 MB)
Individual evidence
- ↑ DIN 55943 . In: German Institute for Standardization e. V. (Ed.): Colorants 1 . 7th edition. DIN-Taschenbuch 49.Berlin, Vienna, Zurich 2012, ISBN 978-3-410-23202-5 , pp. 509 .
- ↑ a b c Wolfgang Glöckner, Walter Jansen, Rudolf G. Weißenhorn (eds.): Handbook of experimental chemistry, secondary level II. Volume 10, Aulis Verlag Deubner & Co. KG, Cologne 2008, ISBN 978-3-7614-2388-2 , Pp. 304-309.
- ^ A b c Christian-Herbert Fischer: Historical dyes . In: Spectrum of Science . No. 10, 1997, p. 104 ff.
- ↑ Ullmann's Encyclopedia of Technical Chemistry . 4th edition. Volume 11, keyword: dyes, natural. P. 103.
- ^ Karl Aloys Schenzinger: Aniline. Zeitgeschichte-Verlag Berlin, 1937, OCLC 6866470 .
- ↑ George B. Kauffman: Pittacal - The first synthetic dyestuff. In: Journal of Chemical Education. 54, 1977, p. 753, doi: 10.1021 / ed054p753 .
- ^ Karl Huebner: History: 150 years of Mauvein . In: Chemistry in Our Time . tape 40 , no. 4 , 2006, p. 274–275 , doi : 10.1002 / ciuz.200690054 .
- ↑ Hans Joachim Störig : Small world history of science 2nd 4th edition. Fischer Taschenbuch, 1982, ISBN 3-596-26399-9 , pp. 136-137.
- ^ A b Hermann Raaf: Organic chemistry in the test glass. 13th edition, Kosmos Verlag, Stuttgart 1975, ISBN 3-440-04266-9 , p. 186.
- ^ Joachim Rudolf: Knauer's book of modern chemistry. Th. Knauer Nachf., Munich / Zurich 1975, ISBN 3-426-00381-3 , pp. 257-263.
- ↑ a b c 1865–1901: The Age of Colors . basf.com. Retrieved November 9, 2018.
- ↑ Renate Kaiser-Alexnat: Indigo - The King of Dyes . In: Southeast Asia Magazine . Issue 3, 2008 ( limited preview in Google Book Search).
- ↑ Ernst Bäumler: The red factory . R. Piper GmbH & Co. KG, Munich 1988, ISBN 3-492-10669-2 .
- ↑ Bayer AG company history , accessed on November 8, 2018
- ↑ Werner Abelshauser (ed.): The BASF: A company history . 2nd Edition. CH Beck, Munich 2003, ISBN 3-406-49526-5 ( limited preview in the Google book search).
- ^ A b Klaus Hunger (Ed.): Industrial Dyes: Chemistry, Properties, Applications . WILEY-VCH Verlag, Weinheim 2003, ISBN 978-3-662-01950-4 ( limited preview in the Google book search).
- ^ John Griffiths: Functional Dyes. A new trend in dye chemistry . In: Chemistry in Our Time . tape 27 , no. 1 . Wiley-VCH Verlag, Weinheim 1993, p. 21-31 , doi : 10.1002 / ciuz.19930270104 .
- ↑ Functional dyes - From the roots of dye chemistry to high-chemical products . chemanager-online.com. Retrieved November 13, 2018.
- ↑ K. Peter C. Vollhardt: Organic Chemistry. VCH, Weinheim, 1st edition 1988, p. 618, ISBN 3-527-26912-6 .
- ↑ Entry on cyanine dyes. In: Römpp Online . Georg Thieme Verlag, accessed on February 4, 2019.
- ↑ Entry on triarylmethane dyes. In: Römpp Online . Georg Thieme Verlag, accessed on January 14, 2019.
- ^ A b Heinrich Zollinger: Color Chemistry: Syntheses, Properties, and Applications of Organic Dyes and Pigments . 3. Edition. WILEY-VCH Verlag, Weinheim 2003, ISBN 3-906390-23-3 ( limited preview in the Google book search).
- ↑ Entry on sulfur dyes. In: Römpp Online . Georg Thieme Verlag, accessed on January 14, 2019.
- ↑ Kirk-Othmer, Jacqueline I. Kroschwitz: Encyclopedia of Chemical Technology. 5th edition, Vol. 9, 2005, ISBN 978-0-471-48494-3 , p. 349.
- ↑ P. Rys, G. Zollinger: Guide to dye chemistry. 2nd edition, Verlag Chemie, Weinheim 1976, ISBN 3-527-25650-4 , pp. 181, 182.
- ↑ Entry on chromating dyes. In: Römpp Online . Georg Thieme Verlag, accessed on January 23, 2019.
- ↑ Bertram Philipp, Peter Stevens Basic Features of Industrial Chemistry. VCH Verlagsgesellschaft mbH, 1987, ISBN 3-527-25991-0 , p. 321.
- ^ Wittko Francke, Wolfgang Walter: Textbook of organic chemistry. S. Hirzel Verlag, Stuttgart 2004, ISBN 3-7776-1221-9 , p. 684 f.
- ↑ Regulation (EC) No. 1333/2008 of December 16, 2008 on food additives (PDF) , accessed on August 5, 2019.
- ↑ Authorization and use of food additives. Federal Ministry of Food and Agriculture, accessed on August 5, 2019 .
- ↑ H. Zollinger: Chemism of the reactive dyes . In: Angew. Chem. 73, No. 4, 1961, pp. 125-136, doi: 10.1002 / anie.19610730402 .
- ↑ a b c John Griffiths: Functional dyes. A new trend in dye chemistry . In: Chemistry in Our Time. 27, No. 1, 1993, pp. 21-31, doi: 10.1002 / ciuz.19930270104 .
- ↑ Klaus Roth: The chemistry of dazzling discs: CD, DVD & Co. In: Chemistry in our time. 41, No. 4, 2007, pp. 334-345, doi: 10.1002 / ciuz.200700428 .
- ^ Hermann Rath: Textbook of Textile Chemistry . including textile chemical technology. 2nd Edition. Springer-Verlag, Berlin, Heidelberg 1963, ISBN 978-3-662-00065-6 , pp. 455 ( limited preview in Google Book search).
- ^ A b Roland Dittmeyer, Wilhelm Keim, Gerhard Kreysa, Karl Winnacker, Leopold Küchler: Chemical technology, processes and products. Volume 7, Industrial Products, 5th Edition. Wiley-VCH Verlag GmbH & Co. KGaA, 2004, ISBN 3-527-30772-9 , p. 397 ff.