Drying
Under a drying or dehydration or desiccation is generally understood as the removal of fluids from a substance or article, the dry material , by evaporation , evaporation , the use of drying means or in other industrial applications such as chemical. A characteristic of drying is therefore the reduction in moisture ( removal of moisture , dehumidification) through a usually thermal-physical conversion of the liquid (usually a phase change into the gaseous state).
The liquid is usually water , which is why one often speaks of dehydration . However, this term is not congruent with drying, as it also includes mechanical or gravitational dewatering (mechanical dehumidification). It is recommended in technical applications, if possible, connected upstream a mechanical dehumidification, since the mechanical process energy expenditure favorable (approximately respect usually 1 / 10 of the energy costs ) are.
The technical field of application that deals with the drying of materials is drying technology . Apparatus for drying tasks are briefly described under the term dryer .
The basis of drying with air as the drying medium with convection is the vapor pressure of the liquid at a certain temperature and pressure. If the content of the liquid in the atmosphere corresponds to (or exceeds) the vapor pressure, no drying (evaporation) is possible. If there is less liquid, it depends on the driving concentration gradient and the gas exchange (wind, surface ...) how fast the drying proceeds. As a rule, the higher the temperature, the higher the vapor pressure, which promotes drying. With decreasing pressure, the possible proportion of the liquid in the atmosphere increases. For important liquids, especially for water, there are diagrams and tables that can be used for technical control of drying. The most important diagram for this is the Mollier diagram for moist air.
The basis of drying with superheated steam as a medium is the temperature gradient (the so-called superheating) and the concentration gradient between the drying medium and the liquid. The decisive difference to air drying is the lack of diffusion transition. Once the saturated steam temperature has been reached, the evaporating water only needs to change its physical state and not dissolve in the air.
Technical drying processes
The high dependence of the drying speed on the temperature is used in kilns , drying chambers , drying cabinets and tumble dryers . However, the drying processes of dough drying , film drying and spray drying associated with a temperature increase are also used. Further processes are the processes of freeze drying, supercritical drying and microwave drying set out below.
Freeze drying
In freeze-drying, the goods to be dried are first frozen. The water then changes from the solid state directly to the gaseous state - it sublimates . One of the advantages is that the goods to be dried are preserved in the frozen state. The production of freeze-dried soluble coffee is economically important. An exotic example of the use of this gentle drying method is the restoration of historical paper documents after the Elbe flood in 2002 .
Supercritical drying
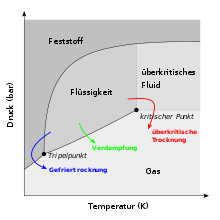
Like freeze drying, supercritical drying also takes a detour around evaporation, but in that the critical point is exceeded with high pressures and temperatures . The basic principle is that the substance is first transferred from the liquid to the supercritical state and then, at a constant temperature, transfers the pressure to the ambient pressure. In contrast to subcritical drying and freeze drying, a phase boundary is not passed, since it is not possible to differentiate between gas and liquid.
Microwave drying
With microwave drying, a wet building fabric is dehumidified directly. This is done by irradiating with high-energy microwaves , which cause the water molecules to move inside the building fabric. The resulting frictional heat dries the building fabric from the inside out. This process is fatal in living organisms.
Condensation process
Condenser dryers remove moisture from the air by cooling it below the dew point and heating it again using a heat recovery register: Moist room air is sucked in by a fan built into the device. The air is passed through a cooling section (the so-called evaporator). The air is then suddenly cooled to such an extent that it falls below its dew point. Since cold air can hardly store moisture, the moisture condenses on the cold surface.
The physical principle of operation can also be observed in summer when you take a cold bottle out of the refrigerator and take it outside - water droplets form on the glass as the ambient temperature cools down on the cold surface. The same principle is used here. In the low temperature range, an ice film forms on this cooling surface. Since almost every air dryer is equipped with an automatic defrosting system, the dryer switches on this defrosting function as required - the ice is liquefied and collects in the container. The dried air passes through a "rewarming section" in the drying device and exits dry and tempered at the front of the device.
The condensate that forms is collected in a water tank or can be discharged directly via a hose line.
Vacuum drying
With vacuum drying, the dry goods are exposed to a negative pressure, which reduces the boiling point and thus leads to evaporation of the water even at low temperatures. The evaporation heat continuously withdrawn from the dry goods must be fed from outside until the temperature is constant. This gentle process is used primarily for heat-sensitive foods and chemicals. This also lowers the equilibrium vapor pressure, which promotes capillary transport.
To improve the drying effect, the material to be dried can be heated, e.g. B. in vacuum drying cabinets , or add a desiccant such as P 2 O 5 for post- drying , with small sample quantities this is done in so-called drying guns .
Adsorption drying
The adsorption dryer is also known as a sorption dryer or rotor dryer. The air to be dried is fed into the sorption dryer by a fan. In the module there is a constantly rotating drying wheel (the rotor - hence the name rotor dryer). This wheel has a honeycomb structure and consists of many axially extending channels. The hygroscopic mode of action of the rotor material removes moisture from the air. The water molecules must be continuously removed ("regenerated") from the rotor. For this purpose, air is sucked in via a second fan, heated to high temperatures and passed over the rotor separately from the other air flows.
Due to the high temperature, the moisture in the rotor evaporates - the heated water vapor is discharged via an air hose. This is the so-called regeneration air flow. The dried air remains in the device - this is fed into the room (dry air flow). Process air is the air flow that is directed from the room into the rotor dryer (i.e. the "humid air flow").
Permeation drying using Nafion membranes
Nafion membrane enables gases to be dried without condensation. Only water vapor is removed from the medium. This means no loss of washable (water-soluble) components such as HCl, Cl 2 , H 2 S, HF, F 2 , SO 2 , NO 2 .
Granulate drying
A container contains granules made up of either salt crystals or chemical substances. With passive granulate drying, the granulate draws moisture from the ambient air. With active granulate drying, the air is sucked in by a fan and passed over the granulate.
The granulate must be renewed at regular intervals. Or you can use regeneration granules, which have to be removed and dried at regular intervals.
Air drying

The air drying with restrictions as - solar drying referred - is an umbrella term for all drying processes at the air , ie under standard atmospheric conditions. In the case of water, the fluid is usually removed by evaporation. When there is no wind, it is a free convection , with wind it is a forced convection. Factors that determine the ultimate drying speed are therefore the temperature , wind speed , air humidity and the effective surface of the dry goods compared to its volume. Air drying is used in a wide variety of areas, for example drying grass to hay , drying wood , paint , varnish , building materials (see clay bricks on the right), sludge and laundry (leash) or drying out bodies of water and puddles . The fluidized bed drying of coarsely crystalline debris is another example of a drying process based on the desiccant air.
Air drying of food
There are different types of air drying for food .
Drying
One type of air drying of food is the preservation form of Dörren s. Water is withdrawn from the food either naturally (air and sun) or in so-called dehydrators through dry air, so that their water content is reduced to a range between 15 and 25 percent. Food can be preserved for a longer period of time, as it spoils or molds much more slowly . The taste is retained and in some cases even intensified.
Drying is used for
- Spices - culinary herbs , pepper , cinnamon
- Fruit - raisins , prunes , banana chips - see also dried fruit
- Legumes - beans , peas , lentils
- Grains - if possible, drying to below 14 percent moisture, typically in roof dryers
- Edible mushrooms
- Meat - Bündnerfleisch , pemmican , biltong - see also dried meat
- Edible fish - stockfish or clipfish
maturation
If food loses a lot of water as a result of a long period of maturation in the air, this is also known as air drying. When the food ripens, fermentation takes place, which prevents microorganisms from multiplying there that would spoil the food:
- Meat - raw ham , salami , Ahle Wurscht and similar sur and smoked products
Air drying of structures
When the temperature rises in summer, the curve of the saturation vapor pressure shifts to higher values. If the component contains condensation water in some areas, the existing vapor pressure p in these areas must correspond to the saturation vapor pressure p s . It comes to dehydration. In the case of single-layer components, for the sake of simplicity, it is assumed that drying out occurs from the center of the moisture penetration zone.
In the case of multi-layered components with condensation at the layer boundaries, the relevant layer boundaries are expected to dry out. If the area is soaked through, the center of the area is selected as the starting point for drying.
In the case of very large temperature differences between outside and inside, drying out can be accompanied by the formation of new condensation in areas further inside. As a result of the reduced drying speed, one speaks of hindered drying.
According to DIN 4108, Part 5, Section 11.2.3, it can be assumed that condensation during the drying period does not need to be taken into account.
As a rule, the simplified climatic conditions according to DIN 4108 Part 3 are used as the basis for the calculation for non-air-conditioned rooms:
| Climatic conditions according to DIN 4108, part 3 | ||
| 1. Thaw period: duration 1440h = 60 days | ||
| Outside climate, inside climate |
−10 ° C +20 ° C |
80% 50% |
| 2nd evaporation period: duration 2160h = 90 days | ||
| 2.1 Wall components and ceilings under undeveloped attic spaces | ||
| Outside climate Inside climate Climate in the condensation area |
+12 ° C +12 ° C +12 ° C |
70% 70% 100% |
| 2.2 Roofs that close off common rooms from the outside air | ||
| Outside air temperature at the roof surface indoor air climate in the condensation area |
+12 ° C +20 ° C +12 ° C temp. According to the temperature gradient from outside to inside |
70% 70% 100% |
Air drying of sewage sludge
For this purpose, the sewage sludge that arises in wastewater treatment and is pre-drained is dried with the help of the natural power of the sun. This type of air drying is called solar sewage sludge drying.
The sludge is applied over a large area in a drying hall. This hall resembles a greenhouse and has a transparent building cladding made of foil, polycarbonate or glass. The drying air in the hall is heated by converting direct and diffuse solar radiation, which heats both the air and the stored sewage sludge. This heating increases the vapor pressure in the sewage sludge compared to the air above and evaporates (see evaporation ) the water from the sewage sludge. A built-in ventilation system in the hall (implemented e.g. by ventilation flaps, fans) ensures a controlled exchange of air. In this way, the moist air is discharged or exchanged from the drying hall.
The possible degree of drying of the sludge depends exclusively on the time. It indicates how much residual moisture there is in the sludge after drying. If you stay in the hall for a long enough time, you will get a degree of dryness in summer of approx. In winter, the specific water withdrawal per m² of surface area is reduced, which means that evaporation is somewhat lower than in summer. There is a low degree of drying of the mud in winter. The drying process produces granules which, on the one hand, can be used as a renewable secondary fuel with a calorific value of 8–11 MJ / kg DM (corresponds to approx. 2–3 kWh / kg DM; conversion: 1 MJ = 0.2778 kWh) in coal power. and cement works (as a substitute for fossil fuels), on the other hand it can also be used as fertilizer. The largest solar sewage sludge drying plant with a drying area of 7,200 m² is currently operated in Nicaragua using the Wendewolf process.
Solvent drying in the chemical laboratory
Organic solvents that are used to carry out chemical syntheses or analyzes are often subject to high purity requirements, e.g. B. an absolute exclusion of water. Different methods are used depending on the type of solvent. Aromatic hydrocarbons are dried by calcium chloride , phosphorus pentoxide or metallic sodium . Alcohols such as methanol and ethanol are dried for. B. by magnesium turnings , tetrahydrofuran is often pre-dried over potassium hydroxide by boiling in a reflux condenser and then fine- dried by pressing in sodium wire.
Typical desiccants and their special features
- Anhydrous calcium chloride is a very popular desiccant because it is inexpensive, has a large water storage capacity, and quickly removes water from hydrocarbons. Calcium chloride anhydrous is used in the form of colorless pellets; After prolonged or improper storage, the pellets can disintegrate into fine calcium chloride dust, this dust can get through filter paper together with the solvent and thus contaminate the solvent. Anhydrous calcium chloride tends to form crystal alcohols, so it cannot be used to dry alcohols.
- Anhydrous sodium carbonate and anhydrous potassium carbonate are useful drying agents for basic solvents. They have a medium water storage capacity and a medium removal rate. These drying agents should not be used with acidic solvents.
- Anhydrous sodium sulfate is a neutral drying agent having a high capacity for water. Drying takes a long time, but sodium sulfate is inexpensive. When water is absorbed, a granular hydrate forms, which can be easily filtered off. Sodium sulfate loses water above a temperature of 32 ° C, so the solvent temperature must be observed.
- Anhydrous magnesium sulfate is a good all-round drying agent having a high capacity for water. Solvents are dried relatively quickly. Since it is used as a fine powder, there is a risk that the products of a synthesis will adsorb on the surface when a product-solvent mixture is to be dried, by washing with fresh, dry solvent it is possible to recover some product. Use a different drying agent for sensitive, acidic solvents or product-solvent mixtures.
- Anhydrous calcium sulfate is a neutral, good all-desiccant with a low water storage capacity but quick recording. It is sold under the brand name "Drierit", which can be purchased with and without an indicator (a cobalt salt).
See also
literature
- Achim Samwald: Drying: fruits, vegetables, herbs . 4th, revised. Edition. Eugen Ulmer, 2007, ISBN 978-3-8001-4922-3 .
Web links
- How air dehumidification works through adsorption
- Notes on drying food
- Examples of calorific values (dry matter)
- Use of secondary fuels (PDF; 1.0 MB)
Individual evidence
- ↑ Stefan Lechtenböhmer, Sabine Nanning, Bernhard Hillebrand, Hans-Georg Buttermann: Use of secondary fuels . Implementation of the inventory plan and national independent review of the emission inventories for greenhouse gases, sub-project 02. Ed .: Federal Ministry for the Environment, Nature Conservation and Nuclear Safety. 2007, ISSN 1862-4804 ( kobv.de [PDF; accessed on April 29, 2011]).
- ↑ Examples of calorific values (dry matter) .
- ↑ Procedure info .
- ↑ Solar sewage sludge drying in Managua ( Memento of the original from June 26, 2013 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. (PDF; 126 kB).
- ^ H. Schick: Cleaning and drying organic solvents . In: C. Weygand, G. Hilgetag: Organic-chemical experimental art . Johann Ambrosius Barth Verlag, Leipzig 1970, pp. 1128–1137.
- ↑ a b c d e f g h i j k l m n Joaquín Isac-García, José A. Dobado, Francisco G. Calvo-Flores, Henar Martínez-García: Experimental organic chemistry: laboratory manual . 1st edition. Academic Press, London, ISBN 978-0-12-803935-9 .
- ↑ Zeno: Lexicon entry on "crystal alcohol". Meyers Großes Konversations-Lexikon, Volume 11 ... Retrieved May 22, 2019 .