Tetrahydrofuran
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Tetrahydrofuran | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 4 H 8 O | |||||||||||||||
| Brief description |
colorless, ethereal-smelling liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 72.11 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.8892 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
−108.39 ° C |
|||||||||||||||
| boiling point |
65.81 ° C |
|||||||||||||||
| Vapor pressure |
|
|||||||||||||||
| solubility |
miscible with water, ethanol , acetone and diethyl ether |
|||||||||||||||
| Dipole moment |
1.63 D |
|||||||||||||||
| Refractive index |
1.4070 |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
DFG / Switzerland: 50 ml m −3 or 150 mg m −3 |
|||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Tetrahydrofuran (THF) is an organic solvent and belongs to the class of ( cyclic ) ethers .
Presentation and extraction
There are various production processes for the manufacture of tetrahydrofuran. The most frequently used process is the Reppe process , which was developed in the 1930s . Starting from acetylene 1 and formaldehyde , 2-butyne-1,4-diol ( 2 ) is initially formed. After its hydrogenation to 1,4-butanediol ( 3 ), the THF 4 is obtained by an acid-catalyzed cyclization .
Another production process starts from 1,3-butadiene ( 5 ). This is oxidatively at 80 ° C and 3 MPa with acetic acid over a palladium - tellurium - catalyst to 2-butene-1,4-diol diacetate ( 6 implemented). After hydrogenation to 1,4-butanediol diacetate ( 7 ) and targeted hydrolysis of the ester 8 , the THF 4 is formed. Alternatively, the process can lead to 1,4-butanediol as the end product.
THF can also be obtained by hydrogenating furan . A more recent synthesis is based on the gas phase hydrogenation of maleic acid dimethyl ester . A reaction sequence runs through the intermediate stages dimethyl succinate , γ-butyrolactone and 1,4-butanediol .
properties
Physical Properties




Tetrahydrofuran is a colorless, flammable liquid with an ethereal odor. It is fully miscible with water up to a temperature of 71.8 ° C, above this temperature a small forms a miscibility gap , which joins at 137.1 ° C again. Mixing with water takes place with a contraction in volume . THF is infinitely miscible with alcohols, ketones and ethers.
With a water content of 19.9 mol%, an azeotropic boiling point of 63.8 ° C. is observed at atmospheric pressure . In the case of alcohols, azeotropic phase diagrams are only observed with methanol and ethanol at atmospheric pressure. The phase diagrams with higher alcohols such as 1-propanol and 2-propanol are zeotropic . The azeotropic boiling points at atmospheric pressure are 60.7 ° C. for methanol with a THF content of 49.7 mol% and 65.7 ° C. for ethanol with a THF content of 85 mol%. These azeotropes are clearly pressure-dependent and disappear for ethanol at lower pressures. Further azeotropes are made with the solvents n-hexane at 63 ° C. with a THF content of 50% by mass , with cyclohexane at 60 ° C. with a THF content of 97% by mass and with acetone at 64 ° C. with a THF content formed by 8 Ma%.
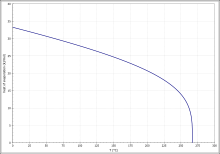
The vapor pressure curve (Fig. 4) can be calculated in the temperature range from 296 K to 373 K with the Antoine equation as log 10 ( p ) = A - ( B / ( T + C )) ( p in bar, T in K) Describe A = 4.12118, B = 1202.942 and C = −46.818.
The temperature dependency of the enthalpy of vaporization (Fig. 5) results from the equation Δ V H 0 = A exp (−β T r ) (1 − T r ) β (Δ V H 0 in kJ / mol, T r = ( T / T c ) reduced temperature) with A = 46.11 kJ / mol, β = 0.2699 and T c = 540.2 K in the temperature range between 302 K and 339 K.
The most important thermodynamic properties are listed in the following table:
| property | Formula symbol | Value (remark) |
|---|---|---|
| Standard enthalpy of formation | Δ f H 0 (g) | −184.2 kJ mol −1 |
| Standard entropy |
S 0 (l) S 0 (g) |
203.9 J mol −1 K −1 (liquid) 301.7 J mol −1 K −1 (gas) |
| Enthalpy of combustion | Δ c H 0 (l) | −2505.8 kJ mol −1 |
| Heat capacity | c p | 124.1 J mol −1 K −1 (as a liquid at 25 ° C) 1.72 J g −1 K −1 (as a liquid at 25 ° C) |
| Enthalpy of fusion | Δ f H 0 | 8.540 kJ mol −1 (at the melting point) |
| Entropy of fusion | Δ f S 0 | 51.8 kJ mol −1 (at the melting point) |
| Enthalpy of evaporation | Δ v H 0 | 29.8 kJ mol −1 (at normal pressure boiling point) |
| Triple point |
T triple p triple |
164.76 K 101.33 kPa |
| Critical temperature | T c | 268 ° C |
| Critical pressure | p c | 51.9 bar |
| Critical volume | V c | 0.225 l mol −1 |
| Acentric factor | ω c | 0.22535 |
Tetrahydrofuran forms a solid hydrate with water with the composition THF · 16.9H 2 O, which melts at 5 ° C.
Safety-related parameters
Tetrahydrofuran forms highly flammable vapor-air mixtures. The compound has a flash point of −20 ° C. The explosion range is between 1.5% by volume (46 g / m 3 ) as the lower explosion limit (LEL) and 12.4% by volume (370 g / m 3 ) as the upper explosion limit (UEL). A correlation of the explosion limits with the vapor pressure function results in a lower explosion point of −23 ° C and an upper explosion point of 13 ° C. The limit gap width was determined to be 0.83 mm. This results in an assignment to explosion group IIB. With a minimum ignition energy of 0.54 mJ, the tetrahydrofuran vapor-air mixtures are extremely ignitable. The ignition temperature is 230 ° C. The substance therefore falls into temperature class T3. The electrical conductivity is rather low at 4.6 · 10 −8 S · m −1 .
According to the dangerous goods regulations , tetrahydrofuran is assigned to class 3 (flammable liquids) with packaging group II (medium hazard) (label: 3).
Chemical properties
Like many ethers, THF also forms a peroxide through autoxidation when left to stand in air for a long time and when exposed to light . This can remain as a highly explosive residue when the THF is distilled off. It is therefore advisable to carry out a peroxide test before each distillation of THF . THF mixed with peroxide should be disposed of for safety reasons.
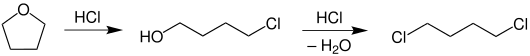
When heated in the presence of hydrochloric acid , the ether is easily cleaved with formation of 4-chlorobutanol and later 1,4-dichlorobutane .
use
Tetrahydrofuran is used as a solvent for PVC , polystyrene , polyurethanes , cellulose nitrate , adhesives and paints ; it is an intermediate in the manufacture of polyamides , polyester and polyurethane, and it is used to produce tetrahydrothiophene and pyrrolidine . It is an important precursor for polytetrahydrofuran . Due to its donor effect , it is used as a solvent in numerous organic reactions .
In addition to diethyl ether , tetrahydrofuran is one of the most important solvents for reactions with basic and neutral reactants , as it has good dissolving properties and is largely inert. In reactions with strongly ( Lewis ) acidic reactants, one must expect ether cleavage. Tetrahydrofuran often forms acid-base adducts with weaker (Lewis) acidic reactants.
A related cyclic ether is 1,4-dioxane .
Risk assessment
Tetrahydrofuran was included in the EU's ongoing action plan ( CoRAP ) in 2012 in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. Tetrahydrofuran uptake was caused by concerns about its classification as a CMR substance, consumer use , worker exposure , high (aggregated) tonnage and widespread use. The reassessment took place from 2013 and was carried out by Germany . A final report was then published.
The International Agency for Research on Cancer (IARC) classified tetrahydrofuran as possibly carcinogenic in 2017.
Web links
- Safety data sheet tetrahydrofuran in the hazardous substance information system of the BG RCI and the BGHM
Individual evidence
- ↑ a b c d e f g h i j k l m Entry on tetrahydrofuran in the GESTIS substance database of the IFA , accessed on July 31, 2017(JavaScript required) .
- ↑ a b The Merck Index . An Encyclopaedia of Chemicals, Drugs and Biologicals. 14th edition. 2006, ISBN 0-911910-00-X , p. 1585.
- ↑ a b c d e f B. V. Lebedev, IB Rabinovich, VI Milov, V. Ya. Lityagov: Thermodynamic properties of tetrahydrofuran from 8 to 322 K. In: J. Chem. Thermodyn. 10 (4), 1978, pp. 321-329. doi: 10.1016 / 0021-9614 (78) 90064-2 .
- ↑ a b c d Yoshio Yoshikawa, Akira Takagi, Masahiro Kato: Indirect Determination of Vapor-Liquid Equilibria by a Small Ebulliometer. Tetrahydrofuran-Alcohol Binary Systems. In: J. Chem. Eng. Data . 25 (4), 1980, pp. 344-346. doi: 10.1021 / je60087a017 .
- ↑ a b c Entry on tetrahydrofuran. In: Römpp Online . Georg Thieme Verlag, accessed on September 29, 2014.
- ↑ Entry on tetrahydrofuran in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Schweizerische Unfallversicherungsanstalt (Suva): Limits - Current MAK and BAT values (search for 109-99-9 or tetrahydrofuran ), accessed on November 2, 2015.
- ↑ a b c H. Müller .: Tetrahydrofuran. In: Ullmann's Encyclopedia of Technical Chemistry . Wiley-VCH Verlag, Weinheim 2005. doi : 10.1002 / 14356007.a26_221
- ↑ T. Onoda: US 3,922,300, 1975 (Mitsubishi Chem.).
- ↑ Yoshinao Nakagawa, Keiichi Tomishige: Total hydrogenation of furan derivatives over silica-supported Ni-Pd alloy catalyst. In: Catalytic Comm. 12, 2010, pp. 154-156. doi: 10.1016 / j.catcom.2010.09.003 .
- ↑ D. Starr, RM Hixon: Tetrahydrofuran In: Organic Syntheses . 16, 1936, p. 77, doi : 10.15227 / orgsyn.016.0077 ; Coll. Vol. 2, 1943, p. 566 ( PDF ).
- ^ Bourguignon: In: Chem. Zentralblatt. 1908, I, 1630.
- ↑ S. Marz, SP Müller, B. Kraushaar-Czarnetzki: Process intensification in the gas phase conversion of dimethyl maleate to tetrahydrofuran. In: Chem. Ing. Techn. 85, 2013, pp. 535-539. doi: 10.1002 / cite.201200230 .
- ↑ J. Matous, J. Hrancirik, JP Novak, J. Sobr: Liquid-liquid equilibrium in the system water-tetrahydrofuran. In: Collect. Czech. Chem. Commun. 35, 1970, pp. 1904-1905. doi: 10.1135 / cccc19701904 .
- ↑ J. Matous, JP Novak, J. Sobr, J. Pick: Liquid-liquid equilibrium in the system water-tetrahydrofuran (2). In: Collect. Czech. Chem. Commun. 37, 1972, pp. 2653-2663. doi: 10.1135 / cccc19722653 .
- ↑ Dortmund database .
- ↑ W. Hayduk, H. Laudie, OH Smith: Viscosity, freezing point, vapor-liquid equilibriums, and other properties of wässrige-tetrahydrofuran solutions. In: J. Chem. Eng. Data . 18 (4), 1973, pp. 373-376. doi: 10.1021 / je60059a027 .
- ↑ E. Brunner, AGR Scholz: Isobaric vapor-liquid equilibria of the tetrahydrofuran / ethanol system at 25, 50, and 100 kPa. In: J. Chem. Eng. Data 29 (1), 1984, pp. 28-31. doi: 10.1021 / je00035a011 .
- ^ IM Smallwood: Handbook of Organic Solvent Properties. Arnold, London 1996, ISBN 0-340-64578-4 , p. 217.
- ^ DW Scott: Tetrahydrofuran: vibrational assignment, chemical thermodynamic properties, and vapor pressure. In: J. Chem. Thermodyn. 2 (6), 1970, pp. 833-837. doi: 10.1016 / 0021-9614 (70) 90026-1 .
- ^ A b V. Majer, V. Svoboda: Enthalpies of Vaporization of Organic Compounds: A Critical Review and Data Compilation. Blackwell Scientific Publications, Oxford 1985, p. 300.
- ^ AS Pell, G. Pilcher: Measurements of heats of combustion by flame calorimetry. Part 3.-Ethylene oxide, trimethylene oxide, tetrahydrofuran and tetrahydropy. In: Trans. Faraday Soc. 61, 1965, pp. 71-77. doi: 10.1039 / TF9656100071 .
- ^ GA Clegg: Thermodynamics of Polymerization of Heterocyclic Compounds. II. The heat capacity, entropy, enthalpy and free energy of polytetrahydrofuran. In: polymer . 9, 1968, pp. 501-511. doi: 10.1016 / 0032-3861 (68) 90060-8 .
- ↑ RC Cass, SE Fletcher, CT Mortimer, HD Springall, TR White: Heats of combustion and molecular structure. Part V. The mean bond energy term for the CO bond in ethers, and the structures of some cyclic ethers. In: J. Chem. Soc. 1958, pp. 1406-1410. doi: 10.1039 / JR9580001406 .
- ↑ a b M. Costas, D. Patterson: Heat capacities of water + organic solvent mixtures. In: J. Chem. Soc., Faraday Trans. 1 . 81, 1965, pp. 2381-2398. doi: 10.1039 / F19858102381 .
- ↑ a b c K. A. Kobe, AE Ravicz, SP Vohra: Critical Properties and Vapor Pressures of Some Ethers and Heterocyclic Compounds. In: J. Chem. Eng. Data . 1 (1), 1956, pp. 50-56. doi: 10.1021 / i460001a010 .
- ↑ J. Schmidt: Design of safety valves for multi-purpose systems according to ISO 4126-10. In: Chem. Ing. Techn. 83, 2011, pp. 796-812. doi: 10.1002 / cite.201000202 .
- ↑ DG Leaist, JJ Murray, ML Post, DW Davidson: Enthalpies of decomposition and Heat Capacities of ethylene oxide and tetrahydrofuran hydrate. In: J. Phys. Chem. 86, 1982, pp. 4175-4178, doi: 10.1021 / j100218a017 .
- ↑ JB Fenn: Lean flammability limit and minimum spark ignition energy. In: Ind. Eng. Chem. 43, 1951, pp. 2865-2869.
- ↑ HF Calcote, CA Gregory, CM Barnett, RB Gilmer: Spark Ignition - Effect of Molecular Structure. In: Ind. Eng. Chem. 44, 1952, pp. 2656-2662.
- ↑ E. Brandes, W. Möller: Safety-related parameters. Volume 1: Flammable Liquids and Gases. Wirtschaftsverlag NW - Verlag für neue Wissenschaft, Bremerhaven 2003.
- ↑ Technical rule for hazardous substances TRGS 727, BG RCI leaflet T033 Avoidance of ignition hazards due to electrostatic charges , status August 2016, Jedermann-Verlag Heidelberg, ISBN 978-3-86825-103-6 .
- ^ Author collective: Organikum . 22nd edition. Wiley-VCH, 2004, ISBN 3-527-31148-3 .
- ↑ European Chemicals Agency (ECHA): Substance Evaluation Conclusion and Evaluation Report .
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): Tetrahydrofuran , accessed on March 26, 2019.
- ↑ Yann Grosse, Dana Loomis, Kathryn Z Guyton, Fatiha El Ghissassi, Véronique Bouvard, Lamia Benbrahim-Tallaa, Heidi Mattock, Kurt Straif: Some chemicals that cause tumors of the urinary tract in rodents. In: The Lancet Oncology . 18, 2017, pp. 1003-1004. doi: 10.1016 / S1470-2045 (17) 30505-3 .