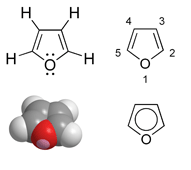
Furan
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Furan | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 4 H 4 O | |||||||||||||||
| Brief description |
colorless liquid with a chloroform-like odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 68.08 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.94 g cm −3 |
|||||||||||||||
| Melting point |
−86 ° C |
|||||||||||||||
| boiling point |
32 ° C |
|||||||||||||||
| Vapor pressure |
|
|||||||||||||||
| solubility |
poor in water (10 g l −1 at 20 ° C) |
|||||||||||||||
| Refractive index |
1.421 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Authorization procedure under REACH |
particularly worrying : carcinogenic ( CMR ) |
|||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−62.3 kJ / mol |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Furan (furfuran) is an organic liquid that is poorly soluble in water from the group of oxygen heterocycles or enol ethers.
history
The name furan is derived from the Latin word furfur for bran . The chemist Carl Wilhelm Scheele produced furan-2-carboxylic acid ( pyrogenic acid ) as the first furan derivative in 1780 . In 1831 Johann Wolfgang Döbereiner reported on another important derivative, the furfural , nine years later the Scottish chemist John Stenhouse was able to characterize it. The chemist Heinrich Limpricht produced furan in 1870 . He mistakenly called it tetraphenol .
Studies on rats suggest a genotoxic effect. In rats, a dose of> 2 mg / kg body weight leads to a greatly increased tumor rate. The estimated daily intake for humans is 1.23 µg / kg body weight. This represents a small safety margin. The World Health Organization (WHO) has classified furan as possibly carcinogenic for humans. The exact effect in the human body has yet to be clarified.
Emergence
Furan is found in coffee, among other things, and is formed there as a decomposition product from furan-2-carboxylic acid and as a result of Maillard reactions from amino acids or carbohydrates . The furan content is directly dependent on the brewing method used. The concentrations found are between 18 and 88 µg / l in brewed coffee.
Extraction and presentation
Furan is technically produced from furfural (furan-2-carbaldehyde). This can by distillation of bran with sulfuric acid are obtained. For decarbonylation (splitting off of carbon monoxide ) the furfural is heated to 400 ° C together with zinc oxide and chromium (III) oxide . In an alternative synthesis route, the furfural is first oxidized with oxygen to form furan-2-carboxylic acid and then converted to furan by decarboxylation . Another possibility for the synthesis of furan and its derivatives is the reaction of 1,4-dicarbonyl compounds (butanedial is required for furan) with dry HCl gas or with phosphorus (V) oxide .
properties
Furan is an aromatic : a five-membered ring with oxygen as a heteroatom , in which six π electrons are delocalized over the ring. Four electrons come from the two double bonds and two electrons from the p orbital of the sp 2 -hybridized oxygen atom. Thus it belongs to the heteroaromatics and to the heterocycles . However, the aromatic character is weaker than that of pyrrole and thiophene .
Furan has a low boiling point (32 ° C) and a high vapor pressure even at room temperature . It is flammable and - because of the high vapor pressure - extremely flammable.
Safety-related parameters
Furan forms highly flammable vapor-air mixtures. The compound has a flash point at −36 ° C. The explosion range is between 2.3% by volume (64 g / m 3 ) as the lower explosion limit (LEL) and 14.3% by volume (405 g / m 3 ) as the upper explosion limit (UEL). The limit gap width was determined to be 0.68 mm. This results in an assignment to explosion group IIB. The ignition temperature is 390 ° C. The substance therefore falls into temperature class T2.
use
An important reaction of furan is hydrogenation to the cyclic ether tetrahydrofuran , which is often used as a solvent .
Derivatives
Furan derivatives occur in flavors and fragrances and act as pheromones in nature . Furan fatty acids are synthesized from fatty acids by various plants and can be detected in a large number of organisms.
Some carbohydrates ( sugars ) form similar structures , these are called furanoses , but do not contain any double bonds in the ring.
See also
- Benzofuran , furan with a condensed benzene ring .
- Dibenzofuran , the basic structure of the class of polychlorinated dibenzofurans .
- Furanocoumarins
- Pyrrole , the equivalent of furan, which contains nitrogen instead of oxygen .
- Tetrahydrofuran (THF), the fully hydrogenated equivalent of furan and a common solvent .
- Thiophene , the sulfur equivalent of furan.
- 2,5-dimethylfuran
Individual evidence
- ↑ a b Entry on furan. In: Römpp Online . Georg Thieme Verlag, accessed on June 7, 2014.
- ↑ a b c d e f g h i j k l m n o Entry on furan in the GESTIS substance database of the IFA , accessed on November 22, 2016(JavaScript required) .
- ↑ Furan data sheet from Sigma-Aldrich , accessed on April 2, 2011 ( PDF ).
- ↑ Entry on furan in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Entry in the SVHC list of the European Chemicals Agency , accessed on July 16, 2014.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-25.
- ↑ Alexander Senning. Elsevier's Dictionary of Chemoetymology . Elsevier, 2006. ISBN 0-444-52239-5 .
- ↑ H. Limpricht: Ueber das tetraphenol C 4 H 4 O . In: Reports of the German Chemical Society . tape 3 , no. 1 , 1870, p. 90-91 , doi : 10.1002 / cber.18700030129 .
- ^ Ernest Harry Rodd: Chemistry of Carbon Compounds: A Modern Comprehensive Treatise . Elsevier, Amsterdam, New York 1971 (English).
- ↑ Carolin Neuwirth, Pasquale Mosesso, Gaetano Pepe, et al .: Furan carcinogenicity: DNA binding and genotoxicity of furan in rats in vivo. In: Molecular nutrition & food research. Volume 56, number 9, September 2012, pp. 1363-1374, doi : 10.1002 / mnfr.201200226 , PMID 22865590 , PMC 3892142 (free full text).
- ↑ Sabrina Moro, James Kevin Chipman, Jan-Willem Wegener, et al .: Furan in heat-treated foods: Formation, exposure, toxicity, and aspects of risk assessment . In: Molecular Nutrition & Food Research, May 29, 2012, doi : 10.1002 / mnfr.201200093
- ↑ Health risk from the pollutant furan in coffee? “ZDFzoom” documentation represents the current state of research , September 25, 2012.
- ^ Furan - test results of the Bavarian State Office for Health and Food Safety , 2009.
- ↑ Eberhard Breitmaier, Günther Jung: Organic chemistry . 4th edition, Thieme, Stuttgart 2001, ISBN 3-13-541504-X , p. 644.
- ^ E. Brandes, W. Möller: Safety-related parameters - Volume 1: Flammable liquids and gases , Wirtschaftsverlag NW - Verlag für neue Wissenschaft GmbH, Bremerhaven 2003.