Dibenzofuran
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
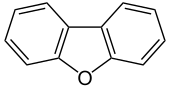
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Dibenzofuran | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 12 H 8 O | ||||||||||||||||||
| Brief description |
colorless, blue fluorescent needles |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 168.19 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.3 g cm −3 (20 ° C) |
||||||||||||||||||
| Melting point |
82 ° C |
||||||||||||||||||
| boiling point |
285 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Dibenzofuran is a heterocyclic aromatic organic compound that consists of a furan ring in the middle and two benzene rings fused to it . At each of the outer carbon atoms , a is in each case hydrogen atom bound. Dibenzofuran is an aromatic ether .
use
Dibenzofuran is found in some hot bath mixes and candle masses and is used to make biphenyls . There are also natural derivatives such as usnic acid .
Dibenzofurans
Under dibenzofurans can understand also a whole class of substances, in which the hydrogen atoms are replaced by other atoms or groups. For example, in 2,3,7,8-tetrachlorodibenzofuran (TCDF), the hydrogen atoms on carbon atoms 2, 3, 7 and 8 have been replaced by chlorine atoms . Polychlorinated dibenzofurans are very toxic substances with properties similar to dioxins .
The dibenzofuran TCDF (2,3,7,8-tetrachlorodibenzofuran) is the dibenzofuran equivalent of dibenzodioxin and Sevesogift's 2,3,7,8-tetrachlorodibenzodioxin .
See also
- Furan , the equivalent without condensed benzene rings .
- Benzofuran , the equivalent with only one condensed benzene ring.
Individual evidence
- ↑ a b c d Entry on dibenzofuran. In: Römpp Online . Georg Thieme Verlag, accessed on April 4, 2014.
- ↑ a b c d e Entry on dibenzofuran in the GESTIS substance database of the IFA , accessed on January 18, 2020(JavaScript required) .
