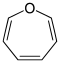
Aromatics
| Most important aromatic: benzene |
 Mesomeric boundary structures of benzene with six π electrons in delocalized double bonds , one of the simplest aromatic compounds. (Note: the presentations above and below are equivalent.) |
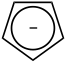
 The delocalized electrons and the equality of the bonds of the benzene molecule are symbolized by a circle. |
 Historic Kekulé benzene formula from the original publication. |
Aromatic compounds , or aromatics for short , are a class of substances in organic chemistry . Its name comes from the aromatic smell of the compounds in this class of substances that were first discovered.
Aromatic molecules have at least one ring system which, according to Hückel's rule , contains a number of 4n + 2 (n = 0,1,2, ...) delocalized electrons in conjugated double bonds, free electron pairs or unoccupied p-orbitals . This delocalization leads to a special bond system in which a distinction cannot be made between single and double bonds in the ring. In simple, symmetrical ring systems such as benzene , all bonds are therefore identical. In structural formulas, all mesomeric boundary structures are shown for clarity or the single bonds are provided with a (sometimes dashed) ring, which symbolizes the delocalized electrons. Compared to non-aromatic double bond systems, aromatics are lower in energy and therefore less reactive . In particular, they are not prone to addition reactions .
Aromaticity criteria
Historical definitions
- Benzene is the simplest aromatic compound to which all other aromatics are related by structure. They often have a pleasant, aromatic odor. The name Aromat, from the Greek word 'aroma' = 'fragrance', is derived from this typical smell.
However, the aromatics cannot be defined by the odor, since with a high molar mass or strongly polar substituents often no odor is perceptible.
- Aromatics are polyunsaturated compounds which are relatively inert towards addition to the double bond and which instead enter into a substitution relatively easily directly on a double bond .
This name definition, which allows an experimental distinction, was valid in the 20th century, for example, even before the structure and bonding relationships were clarified. Today a more general definition of electronic structure is usually preferred. The specified properties - in short: substitution instead of addition - are of course characteristic and very important features.
- The aromatic bonding system shows a particular stability, which can be determined, for example, by comparing the enthalpy of hydrogenation of the aromatic with a corresponding non-aromatic and hypothetical reference compound (in the case of benzene, cyclohexatriene) as the resonance energy .
- The resonance frequency of the hydrogen atoms in the nuclear magnetic resonance experiment is characteristic. This manifests itself in a strong downfield shift for protons outside the aromatic system and an upfield shift for protons within the aromatic system.
Definition of aromatics
Necessary, but not sufficient, requirements for an aromatic:
- A cyclic molecule, that is, it has at least one ring, which in many cases is a benzene ring .
- A double bond system fully conjugated through the ring .
They are either- several double bonds , which in hydrocarbons are separated by exactly one single bond (in the special case of arynes , a triple bond can also occur) or
- one or more double bonds that are separated by positively or negatively charged carbon atoms or by heteroatoms .
This condition is synonymous and shorter:
- all atoms of the ring are sp 2 - hybridized .
An aromatic is present if the following conditions are also met:
- The double bond system is planar; In exceptional cases, slight deviations from the level are permitted. For example, in some cyclophanes the benzene unit is deformed in a boat shape at an angle of up to 30 °.
- The number of delocalized electrons must comply with Hückel's rule, i.e. in the conjugate electron system there must be 2 or 6 or 10 or 14 ... electrons:
The Hückel rule established by Erich Hückel is mostly represented by the formula (4n + 2) π electrons (n = 0,1,2,3…), delocalized over all ring atoms of the system. Cyclic conjugated π systems with 4n π electrons (n = 1,2,3…) are called anti-aromatics .
The basic structure of many aromatic compounds is benzene C 6 H 6 . (The Hückel rule is fulfilled here with n = 1: Benzene has 6 π electrons.) Benzene is therefore regarded as one of the simplest aromatic hydrocarbons - especially since the special properties of aromatic compounds were discovered in benzene and its derivatives . Benzene is more stable and therefore less reactive than a hypothetical (i.e. non-producible) cyclohexatriene with localized double bonds.
Aromatic ions

Since, according to Hückel's rule , a planar, cyclic conjugated system with 2 π electrons is also considered an aromatic, cyclopropenylium salts also contain aromatic cations:

The cyclopropenylium ion fulfills the Hückel rule with n = 0 and is therefore one of the smallest possible aromatic compounds in terms of both the number of delocalized electrons and the ring size.
The negatively charged cyclopentadienyl anion , which occurs in metallocenes such as ferrocene, is also aromatic :
As with benzene, n = 1 here.
Aromatic reactions
Most important are substitution reactions , for example
- Electrophilic aromatic substitution , such as sulfonation , nitration , Friedel-Crafts acylation , azo coupling , chlorination , bromination . Special rules apply to the electrophilic secondary substitution on aromatic compounds. Substituents already present on the aromatic "direct" the further substitution into certain positions .
- Nucleophilic aromatic substitution
- Dearomatization : Loss of the aromatic system through appropriate reduction.
Classification of aromatics
criteria
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
There are an enormous number (several million are known) of different aromatic compounds . They can be divided into groups according to various criteria:
- Since aromaticity is only linked to the cyclic delocalization of electrons and is therefore not limited to organic compounds, the phenomenon of aromaticity is also found in purely inorganic compounds. An inorganic aromatic is, for example, borazole B 3 N 3 H 6 , which can be formally derived from benzene C 6 H 6 by replacing the carbon atoms alternately with nitrogen atoms or boron atoms . However, the aromaticity of borazole is much less pronounced than z. B. in the case of benzene (detailed discussion under borazole). The π electron density is strongly localized on the nitrogen atoms and is not evenly distributed over all ring atoms. Although the parent compound still has a planar geometry, the ring in metal carbonyl complexes adopts a wavy conformation in contrast to benzene. The reactivity when the ring is attacked by nucleophiles or electrophiles (in contrast to the inert benzene) is also a sign of clear bond polarization.
- Ring systems that only consist of carbon atoms are called carbocycles . Benzene C 6 H 6 and naphthalene C 10 H 8 belong to the carbocycles. By contrast, like all heterocycles in the ring system, heteroaromatics contain other atoms such as nitrogen, for example in the aromatic pyridine C 5 H 5 N. (Pyridine is formally derived from benzene by replacing a C – H atom group with N.)
- Carbocyclic aromatics (= with a carbon atom aromatic structure) can be divided into (aromatic) hydrocarbons and substituted aromatics (according to the classification of organic compounds). Benzene C 6 H 6 and toluene C 6 H 5 -CH 3 are hydrocarbons, phenol C 6 H 5 -OH and trinitrotoluene TNT C 6 H 2 (NO 2 ) 3 (CH 3 ) are compounds derived therefrom by substitution.
- Another classification is based on the number of aromatic cycles : One of the simplest aromatic compounds, benzene, consists of exactly one ring. Naphthalene C 10 H 8 is a bicycle , it has an aromatic π system with 10 π electrons, which is distributed over the two rings.
- Aromatics with several rings can be divided into those in which the rings have common atoms (fused or fused rings), as in naphthalene C 10 H 8 , or those with separate (isolated) rings, for example biphenyl C 6 H 5 -C 6 H 5 .
- A further classification can be made according to the number of ring atoms in the aromatic system. Six ring atoms are typical, for example in benzene C 6 H 6 . In order to form a closed ring, at least three atoms are necessary, accordingly there are aromatics with three, four, five, seven or more atoms.
- According to the charge of the aromatic system, for example, the cyclopentadienyl anion is simply negatively charged.
Examples of aromatic compounds
Hydrocarbons
Aromatic hydrocarbons are also called arenes. Examples are:
- Benzene C 6 H 6
- Toluene (methylbenzene) C 6 H 5 -CH 3
- Xylene (dimethylbenzene) H 3 C-C 6 H 4 -CH 3
- Mesitylene (trimethylbenzene) C 6 H 3 (CH 3 ) 3
Hydrocarbons with several rings are called polycyclic aromatic hydrocarbons (PAHs), for example:
- Naphthalene C 10 H 8 (2 rings; two six-membered rings)
- Azulene C 10 H 8 (2 rings; five- / seven-ring)
- Anthracene C 14 H 10 (3 rings)
Annulenes , i.e. cyclic hydrocarbons with conjugated double bonds, can have aromaticity. After benzene, [14] -annulene is the smallest aromatic annulene, and annulenes with 18 and 22 carbon atoms are also aromatic.
Aromatic ions
|
||||||||
Typical parent compounds of this class are the cyclopropenylium ion with 2π electrons, the cyclopentadienyl anion, the cycloheptatrienyl cation and the pyrylium ion with 6π electrons. In the case of the pyrylium ion, the oxygen contributes two electrons to the delocalized π system via one of the two free electron pairs. The second lone pair of electrons lies in the plane of the ring and thus does not contribute to the delocalized π system (analogous to the lone pair of electrons on the nitrogen of pyridine).
Benzene derivatives
- Phenol C 6 H 5 -OH
- Nitrobenzene C 6 H 5 -NO 2
- Aniline (aminobenzene) C 6 H 5 -NH 2
- Hydroquinone HO-C 6 H 4 -OH
- Picric acid (2,4,6-trinitrophenol) C 6 H 2 (OH) (NO 2 ) 3
Heteroaromatics
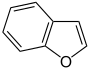
- Furan C 4 H 4 O (five-membered ring with oxygen atom)
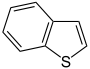
- Thiophene C 4 H 4 S (five-membered ring with sulfur atom)
- Pyridine C 5 H 5 N (six-membered ring with nitrogen atom)
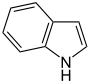
- Pyrrole C 4 H 4 NH (five-membered ring with nitrogen and hydrogen atom)
Halogen aromatics
- Chlorobenzene C 6 H 5 -Cl
- Bromophenols Br-C 6 H 4 -OH
Anti-aromatics
See main article: Antiaromat
|
|||||
Antiaromatics are substances that meet the first three conditions of an aromatic (cyclic, planar, conjugated double bonds), but instead of 4n + 2 π electrons have 4n π electrons . According to the Hückel approximation, anti - aromatics experience little or no stabilization due to the cyclic delocalization. Due to Hund's rule , molecular orbitals degenerate in pairs must each be occupied by one electron. According to the approximation, the anti-aromatics are thus present as highly reactive triplet radicals. The simplest antiaromatic, cyclobutadiene , is only stable at very low temperatures (≤ 20 K ) in a solid matrix. Tri- tert -butylcyclopentadiene, on the other hand, is stable for a few hours at 20 ° C. Interestingly, cyclobutadiene is stable as a ligand in organometallic chemistry , an example being the cyclobutadiene-iron tricarbonyl complex.
Cyclooctatetraene has 8 π electrons. However, it is not planar so that the double bonds are not conjugated. The Hückel rule cannot therefore be applied. 1,3,5,7-Cyclooctatetraene is thus a non-aromatic.
Anti-aromatics are a subset of the non-aromatic alicyclic compounds . The latter also include non-conjugated compounds.
Möbius aromatics
The 1964 by Edgar Heilbronn predicted Möbius Aromaticity implies that the occupied in a cyclic p-conjugated system of π orbitals as a Mobius strip are arranged, ie with a 180 ° rotation. In addition, the π – orbitals are occupied by 4n electrons (where n is a natural number here ). Möbius aromatics are chiral due to the twist . Heilbronner now drew the conclusion that Möbius systems can never be lower in energy than their Hückel counterparts. The problem with him was that he assumed a rotation only for the computation of the Möbius molecules, but not for the Hückelschen ones. A year later Zimmerman had taken a closer look at the problem.
Whether a molecule synthesized by Rainer Herges in 2003 really represents a Möbius aromatic or only has the necessary topology is still a matter of controversy.
Aromatics in nature

Many compounds in nature have aromatic structures. Are ubiquitous in proteins , the amino acids such as tyrosine , tryptophan or phenylalanine . The DNA or RNA , the carriers of genetic information, contain the nucleobase adenine as part of the nucleotide ATP . Vegetable dyes such as the water-soluble flavonoids , the builder lignin of the wood , cofactors of enzymes such as pyridoxal phosphate or pterines are just a few more examples.
In the natural female sex hormones estradiol , estriol and estrone , ring A of the steroid structure is aromatic. In contrast, ring A in the male sex hormones ( androgens ) is not aromatic.
The biochemical synthesis and the breakdown of aromatics is often realized by special enzymes . By breaking down aromatics , microorganisms also return aromatics from inanimate nature, such as pollutants , pesticides or waste from the chemical industry, to the carbon cycle .
See also
literature
- Günther Maier: Aromatic - what does that actually mean? Chemistry in our time , 9th year 1975, No. 5, pp. 131-141, doi: 10.1002 / ciuz.19750090502 .
- Paul von Ragué Schleyer, Haijun Jiao: What is aromaticity? In: Pure and Applied Chemistry . tape 68 , no. 2 , 1996, p. 209-218 , doi : 10.1351 / pac199668020209 .
Web links
- Hückel rule (PDF; 27 kB) ( Memento from August 13, 2007 in the Internet Archive )
Individual evidence
- ↑ August Kekulé: About some condensation products of the aldehyde , Liebigs Ann. Chem. 1872 , 162 (1), pp. 77-124; doi: 10.1002 / jlac.18721620110 .
- ^ FA Carey, RJ Sundberg, Organic Chemistry , VCH, Weinheim, 1995, ISBN 978-3-527-29217-2 .
- ↑ Joachim Buddrus: Fundamentals of Organic Chemistry , 4th edition, de Gruyter Verlag, Berlin, 2011, p. 426, ISBN 978-3-11-024894-4 .
- ↑ E. Heilbronner: Huckel molecular orbitals of Mobius-type conformations of annulenes . In: Tetrahedron Letters . tape 5 , no. 29 , January 1, 1964, p. 1923-1928 , doi : 10.1016 / S0040-4039 (01) 89474-0 .
- ^ Howard E. Zimmerman: On Molecular Orbital Correlation Diagrams, the Occurrence of Möbius Systems in Cyclization Reactions, and Factors Controlling Ground- and Excited-State Reactions. I . In: Journal of the American Chemical Society . tape 88 , no. 7 , April 1, 1966, pp. 1564-1565 , doi : 10.1021 / ja00959a052 .
- ^ Howard E. Zimmerman: Molecular Orbital Correlation Diagrams, Mobius Systems, and Factors Controlling Ground and Excited-State Reactions. II . In: Journal of the American Chemical Society . tape 88 , no. 7 , April 1, 1966, pp. 1566–1567 , doi : 10.1021 / ja00959a053 .
- ↑ D. Ajami, O. Oeckler, A. Simon, R. Herges: Synthesis of a Möbius aromatic hydrocarbon . In: Nature . tape 426 , no. 6968 , 2003, p. 819–821 , doi : 10.1038 / nature02224 .
- ↑ Claire Castro, Zhongfang Chen, Chaitanya S. Wannere, Haijun Jiao, William L. Karney, Michael Mauksch, Ralph Puchta, Nico JR van Eikea Hommes, Paul von Ragué Schleyer Investigation of a Putative Möbius Aromatic Hydrocarbon. The Effect of Benzannelation on Möbius [4n] Annulene Aromaticity . In: J. Am. Chem. Soc. , 2005, 127 , pp. 2425-2432; doi: 10.1021 / ja0458165 .
- ↑ Carsten Schmuck, Bernd Engels, Tanja Schirmeister, Reinhold Fink: Chemistry for Medicine , Pearson Studies, p. 493, ISBN 978-3-8273-7286-4 .