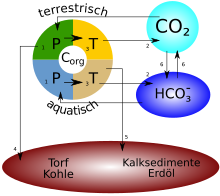
Carbon cycle
The carbon cycle or carbon cycle is understood to mean the system of chemical conversions of carbon-containing compounds in the global systems lithosphere , hydrosphere , earth's atmosphere and biosphere as well as the exchange of these compounds between these earthspheres . The knowledge of this cycle including its sub-processes makes it possible, among other things, to assess human interventions in the climate and thus their effects on global warming and to react appropriately.
System consideration

The "Earth" system is viewed as a closed system . The supply of carbon, for example through meteorites or nuclear chemical processes, and the loss of carbon, for example through space travel, are ignored . Under this condition the total carbon content of the system “earth” can be regarded as constant.
Each of the five subsystems atmosphere , hydrosphere , lithosphere , biosphere and pedosphere forms a carbon store orCarbon reservoir . Such a reservoir has a storage capacity and a stored carbon supply , also called a carbon pool. A reservoir is characterized by the storage forms of carbon. The lithosphere, for example, stores carbon in the form of carbonate rocks ( limestone ), the biosphere in the form of organic carbon compounds and in the calcareous skeletons of animals.
There are between reservoirs Carbon fluxes (also carbon fluxes ). Important parameters that result from the rivers are the length of stay in the reservoir, inflow and outflow (flow rates). If a reservoir R 1 releases more carbon per unit of time to another reservoir R 2 than it absorbs from it, R 1 is oneCarbon source with respect to R 2 while R 2 is a carbon sink . In the context of the carbon cycle, that isCarbon budget , also carbon balance , a budgetary statement of the inflows and outflows of a reservoir. (The term carbon budget - especially in climate policy - can also specifically refer to the absorption capacity of the atmosphere, which remains until the global temperature exceeds a certain limit, see CO 2 budget .)
The terms outlined here can generally be used for any reservoir and also for substances other than carbon. In the context of current climate change, they are often used as a reference reservoir in relation to the atmosphere.
Flow rates can change. For example, it is expected that the absorption capacity of the oceans will decrease with increasing CO 2 concentration in the atmosphere. Reservoirs can swap their roles as a result of the relative change in flow rates, e.g. B. from a carbon sink to a carbon source. For example, the terrestrial biosphere is a carbon source in the northern hemisphere winter and a carbon sink in summer because the land mass in the northern hemisphere is larger. This can be seen in the annual up and down of the Keeling curve , which shows the CO 2 content of the atmosphere.
Carbon storage
The global amount of carbon is 75 million Gt.
the atmosphere
As of 2017, the atmosphere contained around 850 Gt of carbon. That is around 0.001% of the total global carbon. The atmosphere and the biosphere are the smallest carbon stores. The carbon content of the atmosphere is particularly sensitive to changes in flow rates. Due to biochemical processes, however, the atmosphere has the highest carbon flow rates and is therefore part of the short-term cycles.
The predominant carbon compound (and degradation product of other trace gases ) is carbon dioxide (CO 2 ). Since the burning of fossil fuels since the beginning of industrialization has added long-term carbon as CO 2 to the material flows in the environment , the concentration of carbon dioxide in the earth's atmosphere increases . In 2017 it was 406 ml / m³ (corresponds to ppmv); an increase of approx. 130 ppm compared to the pre-industrial value of almost 280 ppm.
Since the beginning of industrialization, a total of approx. 635 Gt of carbon (equivalent to approx. 2300 Gt of CO 2 ) has been released by fossil fuels, almost half of which remained in the atmosphere and a good quarter each was absorbed by the oceans and terrestrial ecosystems (as of 2019) .
Hydrosphere
All bodies of water as well as polar ice caps , ice sheets and glaciers are counted as part of the hydrosphere ( cryosphere ). The hydrosphere contains 38,000 Gt C in the form of dissolved CO 2 , hydrogen carbonate and carbonate ions. This corresponds to 0.045% of the global carbon content. There are also traces of dissolved methane and organic suspended matter .
The carbon dioxide trapped in the ice does not take part in the rapid exchange processes with the atmosphere.
Lithosphere
The lithosphere comprises the outer solid rock strata of the earth and, with 99.95% of the global total carbon, represents the largest carbon reservoir. Due to the low flow rates, the lithosphere is part of the long-term carbon cycle.
- Sediments and the resulting carbonate rocks :
- Carbonates: Calcite CaCO 3 and Dolomite CaMg (CO 3 ) 2 60,000,000 Gt C
- Kerogen (fossil organic substances, for example in oil shale ) 15,000,000 Gt C
- Gas hydrates 10,000 Gt C
- Coal , natural gas , oil 4,100 Gt C
- Pedosphere with humus , peat , sediments, minerals 1,500 Gt
- graphite
Under "normal" conditions, gas hydrates are gaseous substances, on whose molecules with weak binding forces water molecules are attached in a regular arrangement. The attachment of water molecules occurs under certain conditions: solution in water, low temperature, high pressure. The resulting hydrates are mostly solids. Methane hydrates are particularly important for the carbon cycle . The methane molecules are enclosed in cavities in the crystal lattice (see clathrates ). They can be found in marine sediment and in permafrost soil. The methane of the methane hydrates is created by anaerobic microbial decomposition of organic substances. If the water is oversaturated with methane and at temperatures just above freezing point and at high pressure (in the sea from 500 m depth), methane hydrates are formed. By changing the pressure and temperature conditions, larger amounts of methane can be released briefly and get into the atmosphere.
The methane emitted from the deposits can be used by chemoautotrophic archaea under anoxic conditions : Obligatory anaerobic , methane-oxidizing methanosarcinales form acetic acid (ethanoic acid) from methane:
This ethanoic acid is used by the bacterium Desulfosarcina (in a symbiosis with the aforementioned Methanosarcinales) to generate energy in what is known as sulfate respiration :
It is estimated that this symbiosis consumes 0.300 Gt of methane annually, that is more than 80% of the methane produced by archaea in the sediment.
Under oxic conditions methane can be completely oxidized by aerobic , methane-oxidizing bacteria with elemental oxygen (O 2 ) to carbon dioxide and water:
biosphere
Carbon is a relatively rare element in the universe and on earth (percentages mean atomic number ratios):
- Most common elements in the universe: hydrogen (92.7%) and helium (7.2%), ( carbon only 0.008%)
- Most common elements in the earth's crust: oxygen 49%, iron 19%, silicon 14%, magnesium 12.5% ( carbon only 0.099%)
- Most common elements in the human body: hydrogen (60.6%), oxygen (25.7%) and carbon (10.7%)
A development of life on a carbon basis is therefore only possible if living things make use of the global carbon cycle and generate a closed carbon cycle themselves.
Storage forms of carbon in the biosphere are organic substances on the one hand and carbonates (usually calcium carbonate CaCO 3 ) on the other . The building materials for skeletons are of particular importance , such as exoskeletons made from organic materials: chitin in arthropods ( crabs , arachnids , insects ), exoskeletons made from carbonates in molluscs , foraminifera and coccolithophoridae , internal skeletons made from carbonates in corals , which form an average of 0.640 Gt reef limestone annually .
Terrestrial ecosystems contain 800 Gt C, marine 3 Gt C in the biosphere, which corresponds to a total of 0.001% of the total global carbon. Like the atmosphere, the biosphere is one of the smallest carbon stores, but it is the engine of short-term cycles.
Pedosphere
There is at least four times as much carbon in the soil as in the atmosphere. Older publications, on the other hand, assumed that the soil only stores around 1500 billion tons of carbon, i.e. around twice the amount of the atmosphere. Since a large part of the carbon stored in the soil is in permafrost soils , some of it will be released as global warming and thawing of permafrost soils progress .
Processes within the systems
the atmosphere
Mainly physical transport processes take place within the atmosphere. Since the wind causes constant mixing, the CO 2 concentration in the lower layers of the atmosphere is the same everywhere.
CO 2 can only collect on the ground in places that are protected from wind for a long time . Example: carbon dioxide lakes in mines or in caves that are located in volcanically active areas.
Organic trace substances are oxidized to CO 2 (and water) with time constants of one day to ten years .
Hydrosphere
Transport operations
Around 92 Gt of carbon are stored in water reservoirs such as seas or lakes and 90 Gt are released again each year.
- Physical carbon pump: In the sea, sinking water masses briefly transport carbon to great depths of the oceans.
- Biological carbon pump: Sinking marine organisms transport carbon to the bottom of the oceans over the long term.
Chemical reactions and equilibria
There is a chemical equilibrium between the various forms of inorganic carbon (the percentages apply to the conditions T = 10 ° C, pH = 8, salinity 34.3 ‰ - as they exist , for example, in large areas of the oceans):
When the CO 2 concentration in the atmosphere increases slightly, the hydrosphere absorbs more carbon dioxide in order to restore the relative equilibrium. However, larger changes in concentrations change the equilibrium position when the limits of the absorption capacities are reached. Changes in the conditions also change the equilibrium position, for example when the capacity limit for CO 2 in the water drops. Global warming, for example, shifts the equilibrium to the left.
Lithosphere
sedimentation
During sedimentation , poorly soluble inorganic and organic substances slowly sink to the bottom. The sinking speed depends on the particle size and the density of the water and can be very low in undisturbed water. The sedimentation of the calcareous skeletons of the Coccolithophoridae plays a major role in the carbon cycle .
Diagenesis
Diagenesis is the long-term consolidation of loose sediments through chemical, physical and biotic transformations. For example, the limestone skeletons of the microorganisms turn into limestone. Organic deposits are gradually converted into inorganic or other organic substances under certain conditions, such as those found in low-oxygen, warm flat seas. There arise kerogens (for example in oil shale ), tar ( bitumen ), carbon , graphite and oil as well as methane. The diagenesis rate is 0.2 Gt C per year.
metamorphosis
Metamorphosis is the long-term transformation of solid rock due to increased pressure and temperature: the subduction of sediments on the sea floor increases pressure and temperature. The following chemical transformations take place at the interface between lime and silicate sediments ( sand ):
- Calcite turns into calcium silicate ( wollastonite )
- Dolomite turns into soapstone or talc
The CO 2 released in the process dissolves in the liquid magma and is then released in the event of a volcanic eruption or escapes immediately through crevices or volcanoes .
Through tectonic changes, the resulting silicates are transported to the surface and exposed to weathering.
biosphere
Plants bind about 123 Gt of carbon per year, of which 60 Gt are released into the atmosphere through plant respiration , the rest is bound as biomass or carried into the ground. The bacterial decomposition and respiration release about 60 Gt. Humans cause the transfer of about 9 Gt of carbon from the lithosphere into the atmosphere each year , mainly through the burning of fossil fuels, but also through the production of cement , which also releases CO 2 . Within the biosphere there is a flow of carbon from the autotrophic organisms that produce organic matter to the heterotrophic organisms that consume organic matter . Organic material is transported by wind and animals. A closed cycle is only possible through the mediation of atmosphere and hydrosphere.
Carbon sub-cycles
A reservoir is both a source and a sink for carbon fluxes.
A constant exchange takes place between the carbon stores through chemical, physical, geological and biological processes.
Long-term inorganic cycle
These are geochemical processes that can take place over a period of several thousand to billions of years.
Mechanical weathering
Large blocks of stone can be broken up into smaller and smaller portions as a result of thermal stresses ( e.g. frost splintering ), pressure (e.g. glaciers ) and wind and water erosion . This shredded material is transported through flowing waters and deposited again in the estuary . These sediments can again be subjected to metamorphosis by subduction.
Chemical weathering
Weathering of limestone and silicate rock removes CO 2 from the atmosphere through the use of water . The resulting hydrogen carbonate is soluble and remains in the hydrosphere.
- Dolomite weathering:
- Silicate weathering:
SiO 2 (quartz sand) and CaCO 3 (lime) get under the earth's crust through subduction . There they are melted by the heat and react to form silicate and CO 2, which in turn reach the earth's surface through volcanoes. This cycle is called the carbonate-silicate cycle . More CO 2 is bound than is emitted, so that the CO 2 content of the atmosphere is reduced.
If limestone is weathered by other acids, for example sulfuric acid , which can be formed from hydrogen sulfide and sulfur dioxide released by volcanoes through oxidation and reaction with water, CO 2 is released into the atmosphere:
Precipitation
Calcite is precipitated from a saturated calcium hydrogen carbonate solution by increasing the pH value, whereby CO 2 is released:
This reaction is intensified in particular by an increase in the pH value (basic) as a result of CO 2 consumption ( autotrophic organisms) and by high water evaporation. (See also: stalactite , stalagmite , sinter terrace )
Organisms such as mussels , snails and unicellular organisms also precipitate calcite in order to build up skeletons , housings and shells . Small marine organisms ( foraminifera and coccolithophores ), whose exoskeletons sediment after the organisms have died, and thus form calcareous sediments, and corals , which build coral sticks from calcium carbonate, are of particular importance . The CO 2 concentration is significantly increased above coral reefs . All reefs on earth (285,000 km²) are estimated to precipitate 0.64 Gt calcium carbonate per year. Over 0.28 Gt CO 2 are released in the process. However, only part of this is released into the atmosphere (see also: Climate history ).
The cycle is closed again in two ways:
- Through metamorphosis (see above) CO 2 is released into the atmosphere and
- Through tectonic changes, coral stocks, sedimentary rocks and silicate rocks are transported to the surface and thus exposed to weathering.
| process | Flow rates in Gt C per year |
|---|---|
| Diffusion of CO 2 | 91.7 |
| Diffusion of CO 2 | 90 |
| Precipitation of calcite | |
| Weathering of calcite | 0.2 |
| Weathering of calcite and silicate, the CO 2 required for this | 0.2 |
| metamorphosis | 0.2 |
| Volcanism | 0.1 |
| Weathering of silicate |
Long-term organic cycle
These are biochemical processes that are initially rapid, but are coupled with long-term geological processes. Sedimented, organic material is no longer completely broken down under anoxic conditions. Only a small part is converted into CO 2 by anaerobic bacteria . Overlaying with further sediment layers and sinking to greater depths increase pressure and temperature. As a result, the organic biomolecules are converted into kerogen ( e.g. hydrocarbons ) or carbon ( coal ) in the absence of air .
- Petroleum: The kerogen of the rocks (petroleum mother rock) can be transformed into petroleum. Through migration (“migration”) this creates oil deposits. The oldest oil deposits are believed to be 3 billion years old. The main origin of oil was 500 to 1000 million years ago. It originated in lagoon-like, warm shallow seas from sinking dead plants and animals. The gaseous hydrocarbons, especially methane (CH 4 ), can reach the surface of the earth through cracks and crevices in the rock . In the ocean, bacteria can use this gas as an energy source by oxidizing it to CO 2 :
Crude oil that comes to the surface loses its volatile compounds and solidifies to form viscous asphalt , pitch or earth wax (see: Asphalt lake ).
- Coal: Coal deposits emerged from the forest bogs of the Carboniferous period around 359 to 299 million years ago. If coal is transported to the earth's surface by tectonic processes, it can be oxidized to CO 2 by bacteria .
| process | Flow rate in Gt C per year |
|---|---|
| Diffusion and photosynthesis | |
| sedimentation | |
| Diagenesis | |
| Outgassing | |
| bacterial degradation | |
| bacterial methane oxidation |
Short-term organic cycle
These are biochemical processes of assimilation and dissimilation that take place quickly and can be subject to seasonal fluctuations.
- Through photosynthesis of plants, algae ( phytoplankton ) and bacteria, organic substances are produced from CO 2 with the help of light energy.
- Through cell respiration , carbon is oxidized from these substances back to CO 2 with the help of oxygen . Many organisms ferment in a lack of oxygen, whereby the organic substances are mineralized to CO 2 or are incompletely broken down into other organic substances such as methane.
| terrestrial process | Flow rate in Gt C per year |
|---|---|
| Photosynthesis of land plants | 120 |
| Respiration of land plants | 60 |
| Breathing of animals and destructors | 55 |
| Net primary production of land plants | 60 |
| Detritus | 1 |
| marine process | Flow rate in Gt C per year |
| Photosynthesis of the primary marine producers | 103 |
| Respiration of primary marine producers | 92 |
| Breathing of marine consumers and destructors | |
| Detritus | |
| diffusion |
Human interference in the carbon cycle
Causes the increase in the carbon dioxide concentration in the atmosphere

The analysis of boreholes in the Antarctic ice shows that the global carbon dioxide volume concentration in the atmosphere has never exceeded 300 ppm for at least the last 650,000 years. During the ice ages it was 180 ppm lower than during the warm periods . Concentration has risen sharply since the beginning of industrialization . The blue curve in the graphic on the right results from continuous measurements of the Global Atmosphere Watch station (GAW station) Mauna Loa in Hawaii since 1958. It is called the Keeling curve . These measurements result in annual increases in the CO 2 content of the atmosphere corresponding to several gigatons of carbon (Gt C). The anthropogenic emissions listed below are a little more than twice as high. Part is taken up by the oceans acidified by CO 2 , part by land plants that grow more luxuriantly due to CO 2 .
The emissions are currently stagnating (as of 2014 to 2016) at 10 Gt C per year. By far the largest contribution is the burning of fossil fuels ( crude oil , natural gas , coal ), followed by the CO 2 release during cement and steel production , see list of the largest cement manufacturers or steel industry / tables and graphics .
Fossil carbon compounds are used as reducing agents in steel production . In cement production, the CO 2 comes from calcium carbonate, which reacts with clay (aluminum silicate) to form calcium silicate. From the released CO 2 is in the setting part again removed from the air by the formation of calcium carbonate. In contrast, with the lime mortar used previously, CO 2 was quantitatively bound again.
CO 2 is also released during glass production :
- Sodium carbonate reacts with silicon dioxide (sand) to form sodium silicate.
Estimates of new potential carbon sinks
Afforestation
Afforestation and better management (erosion control, selection of species, changes in the use of plantations , conversion of fields into pastureland and other measures) increase the effectiveness of CO 2 consumption through photosynthesis of the cultivated plants. This results in a consumption of 1.202 to 1.589 Gt C per year. (The range of the estimate results from the uncertainty in the estimate of the effect of newly afforested forests, which is 0.197 to 0.584 Gt C per year.) However, this is offset by a release of 1.788 Gt C per year due to slash and burn. The role of the oceans in the global carbon cycle, especially as a carbon sink, has been a. 1990–2002 examined in the international research project JGOFS ( Joint Global Ocean Flux Study ).
Sustainable use of wood
Sustainable use of wood can reduce the increase in the carbon dioxide concentration in the atmosphere and thus the greenhouse effect. By using wood in a sustainably managed forest, the carbon stored in the wood is withdrawn from the atmosphere over a long period of time. Without the use of wood, for example in a natural or primeval forest, the stored carbon is released back into the atmosphere as carbon dioxide through the decomposition of the trees. The forest in Germany stores carbon corresponding to around 8% of the annual carbon dioxide emissions. As a result, increased use of wood, for example in construction or furniture construction, can store carbon from the atmosphere in the long term. A wooden desk stores around 23 kg of carbon, equivalent to around 83 kg of carbon dioxide. A garden bench made of local wood is about half that. By way of comparison: 83 kg of carbon dioxide corresponds to the amount that a German passenger car emits on average to cover a distance of around 520 km. After its useful life, the wood can continue to be kept as a carbon store through recycling, or the stored energy can be used through incineration, whereby just as much carbon dioxide is produced as was previously removed from the atmosphere by its formation.
consequences
Effects on photosynthesis
A carbon dioxide volume concentration of 1 ‰ would be optimal for photosynthesis in terrestrial plants. The increase in the photosynthesis rate is less than expected, however, since the enzyme responsible for the carboxylation ( RuBisCO ) reacts depending on the temperature. As the temperature rises, the rate of carboxylation of the Rubisco decreases. This applies to C3 photosynthesis . Provided that the oxidation rate of the organic substance increases in accordance with the thermochemical principle with a rise in temperature, there is a dynamic positive feedback of both processes with the result of faster rising atmospheric CO 2 contents: rising CO 2 contents in the atmosphere - temperature rise - increase in the oxidation rate with a lower or inadequately increasing rate of carboxylation - increasing CO 2 levels in the atmosphere. This dynamic slows down as the rate of carboxylation and rate of oxidation come closer and closer. If the oxidation rate falls below the carboxylation rate, the atmospheric CO 2 content and temperature decrease. The Rubisco reacts to falling temperatures with higher carboxylation rates. Here, too, there is again a positive feedback with temperatures and CO 2 levels falling more rapidly. The cause of the dynamics can be found in the temperature-dependent bifunctionality of the Rubisco. For the current situation of climate change, the following conclusion can be drawn: Once the anthropogenic dynamics of rising temperature and CO 2 levels in the atmosphere have started to move, a dynamic of its own will develop that cannot be stopped by human hands .
Disruption of the circuits
The increase in the concentration of carbon dioxide in the atmosphere leads to an increased dissolution of CO 2 in seawater. Due to the formation of carbonic acid, the pH value of the water is lowered ( more acidic ). This hinders the biogenic and abiogenic precipitation of lime. As a result, the amount of phytoplankton should decrease and the rate of photosynthesis decrease.
As a result of the lowering of the pH value of rain and water, the weathering of limestone and thus the consumption of CO 2 would have to increase. Since geochemical flow rates are very low, this effect does not play a role in the short term.
Importance for climate change
Without human intervention, a relatively stable steady state equilibrium has been established in the course of the earth's development . Every participant in the cycle releases and absorbs carbon without any significant changes in the distribution of the carbon.
By burning fossil fuels , carbon, some of which has been stored for millions of years, is released into the earth's atmosphere in the form of CO 2 . On the threshold of the 21st century, mankind “produced” around 8.7 Gt C per year. The unstable equilibrium is disturbed. The result is global warming , to which the growing proportion of the greenhouse gas CO 2 in the earth's atmosphere contributes significantly .
In order to counteract this, attempts are being made to develop processes to remove the excess carbon from the atmosphere and store it in the sediment reservoir ( CO 2 sequestration ).
- See also: consequences of global warming
Problems of technical solutions
Solutions to the CO 2 problem are currently being discussed which, although technically feasible, cannot be controlled and the ecological consequential damage cannot be assessed:
- Liquefaction of CO 2 and introduction into underground, gas-tight storage facilities such as non-mineable coal seams , mined salt deposits , deep water-bearing rock layers and exhausted oil and gas fields. The latter is already being used at “ Sleipner ”, a gas and oil drilling rig owned by the Norwegian company Statoil , where weekly 20,000 t of CO 2 are pumped back into the exploited deposits. This method is considered to be relatively safe because the sand layers of the reservoir have held oil and natural gas for millions of years.
- Dumping of frozen CO 2 (so-called dry ice ) in the sea. During the sinking, the dry ice dissolves, which increases the CO 2 concentration and lowers the pH value of the water, which leads to local poisoning of the organisms.
- Liquefaction and injection into the sea at a depth of 600 m. Gas hydrates should form there, which, due to their higher density, should sink to the bottom. Tests showed that although the gas hydrates were formed, they rose to the surface again.
- Liquefaction and injection into the deep sea below 3,000 m. Here the CO 2 would have to remain liquid and form large carbon dioxide lakes in sinks. Tests showed that the CO 2 did not remain liquid, but formed large-volume gas hydrates that could rise again. Since the formation of gas hydrates increases the concentration of the salinity of the surrounding water through the freezing out of freshwater ice, all organisms would be damaged here. In addition, the CO 2 could not be controlled at this depth: It unexpectedly took up a large volume and could not be collected in sinks, as the smallest currents distributed the CO 2 droplets. This version is favored by the US government. A large-scale experiment in the summer of 2002 off the coasts of Hawaii and Norway was stopped for the time being due to massive resistance from residents and various environmental groups.
Remarks
The figures are estimates and can vary greatly depending on the literature used. It is not always clear what is summarized under the respective carbon fluxes. The information is not always complete. This creates problems such as the carbon balance of the terrestrial biosphere: the inflow of 120 Gt C per year due to assimilation is offset by an outflow of only 116 Gt C per year due to dissimilation and detritus formation. This means that the balance lacks 4 Gt C per year.
See also
literature
- Beth N. Orcutt, Isabelle Daniel, Rajdeep Dasgupta (Eds.): Deep Carbon - Past to Present . Oxford University Press, 2019, ISBN 978-1-108-67795-0 , doi : 10.1017 / 9781108677950 (Open Access, with a focus on the lithosphere and geological depth, summarizes the results of the Deep Carbon Observatory ).
- David Archer : The Global Carbon Cycle (= Princeton Primers in Climate ). Princeton University Press, 2011, ISBN 978-0-691-14413-9 (Introduction).
- Practice of science . In: Biology in School . Issue 3/53, April 15, 2004. Aulis Verlag Deubner, Cologne Leipzig.
Web links
- Global Warming: The Ocean and Its Secrets - Magazine for European Research, # 48, February 2006
- Video and multimedia DVD in the loan portfolio of the Baden-Württemberg State Media Center
- Information on video and multimedia DVD on the producer's website
Individual evidence
- ↑ The term carbon pool can also be used synonymously with reservoir.
- ↑ a b Glossary . In: JM Allwood et al. a. (Ed.): Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change . 2014 ( ipcc.ch [PDF]).
- ↑ Martin Kappas: Climatology: Climate Research in the 21st Century - Challenge for Natural and Social Sciences . Springer, 2009, ISBN 978-3-8274-1827-2 , pp. 158-159 , doi : 10.1007 / 978-3-8274-2242-2 .
- ^ Matthias Schaefer: Balance . In: Dictionary of Ecology . Spectrum Academic Publishing House, September 2011.
- ↑ carbon budget. In: Meteorology Glossary. American Meteorological Society, accessed September 7, 2016 .
- ↑ See for example sink. In: Meteorology Glossary. American Meteorological Society, accessed September 7, 2016 .
- ↑ Markus Reichstein: Universally and Everywhere. The terrestrial carbon cycle in the climate system. In: Jochem Marotzke , Martin Stratmann (Hrsg.): The future of the climate. New insights, new challenges. A report from the Max Planck Society. Beck, Munich 2015, ISBN 978-3-406-66968-2 , pp. 123-136, pp. 125-127.
- ^ Robert A. Berner, The carbon cycle and CO 2 over Phanerozoic time: the role of land plants, The Royal Society, 1998
- ↑ Markus Reichenstein: Universally and Everywhere. The terrestrial carbon cycle in the climate system. In: Jochem Marotzke , Martin Stratmann (Hrsg.): The future of the climate. New insights, new challenges. A report from the Max Planck Society. Beck, Munich 2015, ISBN 978-3-406-66968-2 , pp. 123-136, especially pp. 125-129.
- ↑ a b c d US Department of Energy : Carbon Cycling and Biosequestration. Integrating Biology and Climate Through Systems Science. Report from the March 2008 workshop. 2008. PDF (16 MB).
- ↑ Lennart Schada von Borzyskowski, Francesca Severi a. a .: Marine Proteobacteria metabolize glycolate via the β-hydroxyaspartate cycle. In: Nature. 575, 2019, p. 500, doi : 10.1038 / s41586-019-1748-4 .
- ↑ A new piece of the puzzle in the global carbon cycle. In: mpg.de . November 13, 2019, accessed November 21, 2019 .
- ↑ Gavin Schmidt: 650,000 years of greenhouse gas Concentrations . RealClimate.org , 2005.
- ^ Science . Vol. 288, May 2000.
- ↑ wdc-mare.org
- ↑ Important steps towards climate protection: forest - wood - climate . Wood sales fund, Bonn 2009.
- ↑ Corinne Le Quéré , Glen Peters et al .: Global carbon budget 2012 (PDF; 2.6 MB) In: global carbon project . Tyndall Center for Climate Change Research . December 2, 2012. Archived from the original on September 27, 2013. Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. Retrieved April 7, 2013.