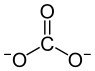
Calcium carbonate
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Calcium carbonate | |||||||||||||||||||||
| other names | ||||||||||||||||||||||
| Molecular formula | CaCO 3 | |||||||||||||||||||||
| Brief description |
colorless and odorless solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 100.09 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
|
|||||||||||||||||||||
| Melting point |
> 825 ° C (decomposition) |
|||||||||||||||||||||
| solubility |
practically insoluble in water (14 mg l −1 at 20 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| MAK |
Switzerland: 3 mg m −3 (measured as respirable dust ) |
|||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| Thermodynamic properties | ||||||||||||||||||||||
| ΔH f 0 |
|
|||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Calcium carbonate (technical language), calcium carbonate or in the German trivial name carbonate of lime , is a chemical compound of the elements calcium , carbon and oxygen with the chemical formula CaCO 3 . As a calcium salt of carbonic acid , it belongs to the group of substances called carbonates . It is a colorless, crystalline solid, the crystal structure of which consists of the ions Ca 2+ and CO 3 2− in a ratio of 1: 1.
Occurrence

Calcium carbonate is one of the most common compounds on earth, especially in the form of sedimentary rocks . It occurs primarily in the form of the mineral calcite (calcite, double spar), which is one of the most common minerals in the earth's crust . It is not only the predominant crystal in the massive limestone, in combination with quartz , barite and fluorite it also forms the bedrock of many ore veins . It can even be the only part of corridors whose thickness ranges from a few centimeters to a few tens of meters. Further modifications of calcium carbonate are the minerals aragonite and vaterite . The name aragonite is derived from the most important occurrence of the mineral in Aragon . It occurs increasingly in the vicinity of marine waters. The reason for this is the magnesium contained in sea water , which favors the formation of aragonite compared to calcite. Along with calcite, aragonite is the most common bio- mineral . It is the inorganic component in mother-of-pearl in mussels, is also often found in pearls and is also found in the shells of marine protozoa and corals . In comparison to calcite and aragonite, vaterite occurs only rarely in nature. It is named after the German chemist and mineralogist Heinrich Vater . In the shells of some snails, all three anhydrate modifications of the compound occur side by side.
In addition, there are two other pseudopolymorphic minerals, monohydrocalcite and ikaite , which are hydrates of calcium carbonate. The unstable monohydrocalcite is the monohydrate of calcium carbonate. The first natural occurrence of monohydrocalcite was described in 1959 and 1964 as an impure sediment in Lake Issyk-Kul in Kyrgyzstan. In living things it occurs in bladder stones of guinea pigs and in the ear stones of some vertebrates. Ikaite is the hexahydrate of calcium carbonate and was first discovered in nature in 1963 in the form of pillars up to 20 meters high on the Ikkafjord in Greenland . The location also gave the mineral its name. The mineral is also unstable at atmospheric pressure above 0 ° C and turns into calcite. Its formation is promoted by magnesium (which is abundantly contained in seawater) and by other additives.
Calcium carbonate is the main component of sedimentary limestone , metamorphic marble and other sedimentary rocks such as oolite or stromatolite . In living nature it occurs in the exoskeleton of crustaceans , corals , mussels , snails and unicellular organisms . In these it is partly also in one of the several known amorphous calcium carbonate phases (ACC).
The first limestone of any significant size was formed by stromatolites over two billion years ago.
Calcium carbonate is widely available in the soil . The most important calcium minerals in our soil are calcite and dolomite [CaMg (CO 3 ) 2 ]. They are released into the ground when carbonate rocks or marls weather.
Contrary to popular belief, the bones and teeth of vertebrates do not contain calcium carbonate, but rather the calcium-containing substances hydroxylapatite (in bones) and fluorapatite in the teeth. However, calcium carbonate occurs in plants, for example. The leaf hairs of the red dogwood are coated with calcium carbonate, which can cause irritation if it comes into contact with sensitive areas of the human skin.
Calcium carbonate has also been detected on the planet Mars .
Modifications in nature

In nature, calcium carbonate forms different rocks that are chemically identical but differ in some ways. Calcite is one of the few minerals that is represented in pure form as a rock former in all three main groups. Because in addition to the sediments chalk and limestone as well as the metamorphite marble, there are also igneous calcium carbonate rocks - the carbonatites .
chalk
Chalk is a fine, microcrystalline sedimentary rock that was formed by the deposition of calcite precipitated by photosynthetic carbonic acid removal and the aragonitic shells of small fossil creatures such as coccoliths of coccolithophores and shells of foraminifera . Chalk appears in numerous locations along the European chalk belt, from Great Britain to France to the island of Rügen in northern Germany, and is mined in places. Sea chalk on the bottom of lakes or in silted lake basins consists almost entirely of precipitated calcite. The blackboard chalk used in school is now mostly no longer made from real chalk, but mainly from plaster of paris ( calcium sulfate ).
limestone
Limestone is also mainly formed by living things and is more solidified than chalk. The calcium deposition occurs either directly or indirectly from the remains of living things, such as snails , mussels , rock-forming hard corals and sponges , which deposit calcium carbonate to build up external or internal skeletons. It is formed indirectly by the fact that living things, especially phototrophic ones , assimilate CO 2 and thus alkalize the environment, which leads to the precipitation of calcium carbonate. The size of the carbonate crystals is between that of chalk and marble. Large limestone deposits can be found, for example, in the Swabian and Franconian Alb , in the Jura , in the Limestone Alps and the Western Alps , in the Himalayas and in many other areas.
marble
Marble is a coarsely crystalline, metamorphic rock that is formed when chalk, limestone or dolomite is recrystallized under the influence of high temperatures and / or high pressures (over 1000 bar). Large marble deposits can be found in Europe, for example, in South Tyrol (Laas), Austria (Gummern), Norway (Molde) or in Carrara , Italy , the home of the pure white Statuario , from which Michelangelo created his sculptures, as well as in North America.
presentation
Synthetic calcium carbonate is referred to as PCC ( English precipitated calcium carbonate ) - in contrast to GCC (English ground calcium carbonate "ground calcium carbonate"). PCC can be made in a number of ways. Well-known processes are precipitation with carbon dioxide, the soda-lime process and the Solvay process , in which PCC is a by-product of ammonia production.
Precipitation with carbon dioxide is the most commonly used process, especially in on-site plants in the paper industry. Clean limestone or quicklime is first slaked into calcium hydroxide (milk of lime) and then fed to the reaction tank as a thin suspension. Carbon dioxide is introduced there until the calcium hydroxide has been completely converted into calcium carbonate. The reaction time can be assessed and controlled by the course of the pH value .
Precipitation takes place at a solids content of around 20%. Different crystal shapes ( crystal morphologies ) and grain distributions can be generated (“grown”) through the process control (temperature, pressure, time, concentration) . The rhombohedral or scalenohedral crystal form is preferred . Because highly pure starting products can be used, the PCCs are particularly white and also have advantages in terms of opacity . Large paper mills are now producing PCC in a "network" by recovering carbon dioxide, which is produced in the form of flue gases during combustion in power plants , by binding it to calcium hydroxide. However, this does not make any contribution to reducing the carbon dioxide concentration in the atmosphere ( climate change ) because natural limestone has to be burned during the previously necessary production of hydrated lime, which releases CO 2 again .
There is an extraordinary calcium carbonate deposit in Villeau , France , where a fossil calcium carbonate precipitate has never solidified for over 30 million years, but has remained loose and thus resembles artificially precipitated PCC in its composition and structure.
In the soda-lime process, calcium carbonate is produced as a by-product in the manufacture of the caustic alkalis sodium hydroxide and potassium hydroxide .
Vaterite precipitates out in the form of microscopic crystals, especially from oversaturated solutions. Monohydrocalcite can be obtained by dewatering with constant suction of the water of the hexahydrate or by additives such as. B. Magnesium, can be synthesized directly in a temperature range of 0 to 40 ° C. Ikait was first produced by Pelouze in 1865 by introducing carbon dioxide into an aqueous calcium oxide / sugar solution. It can also be made by adding a sodium carbonate solution to an ice-cold calcium chloride solution. The grinding of Ikaite leads to the release of the water of crystallization, which in addition to calcite also produces a considerable amount of vaterite.
According to Ostwald's rule of steps, amorphous calcium carbonate is the first phase to precipitate in the crystallization of calcium carbonate. It contains trapped and interstitial water, but the water content can vary and further transforms into one of the anhydrous polymorphic phases of calcium carbonate in aqueous solutions. Various types of additives can stabilize ACC, but stabilized ACC can also be obtained without the aid of additives if it is precipitated at high supersaturation. ACC that is additive-free and precipitated from equilibrated, slightly supersaturated (metastable) aqueous calcium carbonate solutions with the help of a sudden change to a poor solvent for calcium carbonate (e.g. by "quenching" in ethanol ). ACC was first used by Johnston et al. made by mixing concentrated solutions of calcium chloride and sodium carbonate. A method was later found whereby, when carbon dioxide was introduced into a saturated calcium hydroxide solution, initially amorphous calcium carbonate was formed, which then crystallized further to form calcite.
properties
Physical Properties
Calcium carbonate occurs in several anhydrous and also two hydrate modifications as well as other amorphous forms. All are colorless and odorless solids in their pure form. Calcium carbonate breaks down into calcium oxide and carbon dioxide from around 600 ° C, the exact breakdown temperature depending on the existing CO 2 partial pressure.
The most thermodynamically stable and therefore by far the most common calcium carbonate modification under normal conditions is calcite. Like all non-cubic, translucent minerals, it has the special property of birefringence, i.e. splitting an incident light beam into two polarized light beams, which are refracted to different degrees. Under normal conditions, calcite crystallizes in a trigonal structure in the space group R 3 c (space group no. 167) . There is also a high temperature modification (985 ° C at normal pressure) with the space group R 3 m (no. 160) and several high pressure modifications ( Calcite II - Calcite V). At room temperature, calcite II is formed at 1.70 GPa, which then changes to calcite III at 2.15 GPa. Calcite III is stable at room temperature up to 6.16 GPa and only converts to aragonite when the temperature is increased to 345 ° C.
Aragonite crystallizes in an orthorhombic crystal structure with the space group Pmcn (No. 62, position 5) . The calcium ions are approximately at the positions of a hexagonal closest packing, which is distorted by compression along the sixfold axis. Aragonite is dry and stable at room temperature for an indefinite period of time. On the other hand, in solution it slowly transforms into calcite, the morphology of which is dependent on the conditions in the conversion of aragonite. If the transformation takes place in solution, rhombohedral crystals are obtained, while the needle-shaped structure of the aragonite remains when the transformation takes place through heating. The aragonite-calcite transformation is irreversible and does not take place at a specific temperature. When heated to 400 ° C, it takes about three hours to convert to calcite, while at 470 ° C it only takes a few minutes. The entire conversion range is between 387 ° C and 488 ° C. Geologically mineralized aragonite does not tend to convert between 10 and 90 ° C, while synthetically produced aragonite in solution near 90 ° C converts to calcite. However, aragonite is the most stable phase of calcium carbonate above 70 ° C and 1013 hPa and at extreme pressures.
Vaterite is the most unstable anhydrous crystalline form of calcium carbonate. In the dry state, it is stable over a long period of time, but when it comes into contact with water it turns into calcite due to its dissolution and subsequent recrystallization. It is therefore difficult to obtain vaterite from aqueous solutions. The phase transition temperature to calcite depends on the respective synthesis conditions and is in the range from 320 to 460 ° C. It crystallizes in a hexagonal crystal structure with the space group P 6 3 / mmc (No. 194) .
Monohydrocalcite, the monohydrate of calcium carbonate, crystallizes in one of the two trigonal crystal structures with the space group P 3 1 21 (No. 152) or P 3 2 21 (No. 154) . It is unstable and crystallizes within a few hours, immediately when moistened with water, into the anhydrous calcite phase. A decrease in temperature and an increase in hydrostatic pressure stabilize it. No regression to the hexahydrate Ikait can be observed.
The ikaite as a hexahydrate of calcium carbonate crystallizes in the monoclinic space group C 2 / c (No. 15) . The structure contains discrete CaCO 3 ion pairs, each surrounded by a shell of 18 water molecules. It is unstable at atmospheric pressure above 0 ° C, but is also stable at room temperature at pressures of 6 to 7 kbar. Although it sometimes exists at higher temperatures, a conversion to calcite begins at over 5 ° C, which can no longer be stopped even if the temperature is lowered again. Solubility determinations show that Ikaite is more soluble than aragonite and calcite and that, in contrast to the anhydrous polymorphs, its solubility decreases with decreasing temperature.
Amorphous calcium carbonate (ACC) is the name given to modifications of calcium carbonate that do not produce discrete X-ray or electron diffraction reflections. It contains up to one molecule of water per carbonate ion. It is unstable, with low precipitation temperatures stabilizing the amorphous phase during manufacture, while an increase in temperature leads to rapid crystallization. A strongly alkaline mother liquor or the use of suitable additives in the precipitation, including above all magnesium, phosphates and a number of organic substances, also stabilize ACC. The crystallization product depends on the conditions. In the range from 14 to 30 ° C, vaterite and calcite are formed within 3 to 6 hours, while at 60 to 80 ° C aragonite and calcite are formed in 17 to 22 hours. In the temperature range in between (40–50 ° C) all three crystalline anhydrous phases are formed. In the presence of organic substances such as amines, amino acids and carboxylic acids, however, ACC converts to vaterite. Ethylenediamine and sodium aspartate are the best additives for vaterite formation.
| modification | Calcite | Aragonite | Vaterite | monohydroperfluoroalkanes calcite |
Ikait | ACC |
|---|---|---|---|---|---|---|
| formula | CaCO 3 | CaCO 3 | CaCO 3 | CaCO 3 · H 2 O | CaCO 3 • 6H 2 O | CaCO 3 |
| Crystal system |
trigonal | ortho- rhombic |
hexagonal | trigonal | monoclinic | - |
| Space group | R 3 c (No. 167) | Pmcn (No. 62, position 5) | P 6 3 / mmc (No. 194) | P 3 1 (No. 144) | C 2 / c (No. 15) | - (amorphous) |
| Lattice constants Å |
a = 4.991 c = 17.062 γ = 120 ° |
a = 4.959 b = 7.964 c = 5.738 |
a = 4.130 c = 8.490 γ = 120 ° |
a = 10.5536 c = 7.5446 γ = 120 ° |
a = 8.792 b = 8.312 c = 11.021 β = 110.53 ° |
- |
| Density g / cm −3 |
2.71 | 2.93 | 2.65 | 2.43 | 1.83 | |
| stability | stable | stable above 70 ° C |
metastable below 10 ° C |
Water release from 60–80 ° C |
stable below 0 ° C |
unstable
when dehydrated |
Chemical properties
Like all carbonates, calcium carbonate is also sensitive to acids. The most common detection method for carbonate rocks is based on this simple reaction. If you drip a little hydrochloric acid on a limestone, carbon dioxide is released and lets the liquid effervesce.
According to legend, the Carthaginian general Hannibal also made use of this sensitivity to acids when he crossed the Alps with his elephants in 218 BC. To make it easier for the animals to climb the rock, he ordered his soldiers to pour large amounts of vinegar on the compact limestone, whereupon the rock "chemically" weathered and the soldiers could effortlessly break steps into the rock for the elephants.
Calcium carbonate itself is hardly soluble in pure water. The solubility is just 14 milligrams per liter, with the carbonate ion going into solution as hydrogen carbonate ion.
However, in the presence of dissolved carbon dioxide, the solubility increases more than a hundred times. The weathering of limestone is based on this effect, whereby the easily soluble calcium hydrogen carbonate is formed.
Because of its solubility, calcium hydrogen carbonate is a component of most natural waters, depending on the rock in different concentrations. The concentration of calcium carbonate in water in Germany is given as the “ degree of German hardness ” (1 ° dH = 10 mg / liter CaO or 17.85 mg / liter CaCO 3 or 0.18 mmol / l). In France, the unit of measurement “degree of French hardness” is used, with 1 ° fH = 0.1 mmol / l Ca 2+ or Mg 2+ ions. In Switzerland either the direct indication of mmol / l or the French hardness is used.
Most of the natural occurrences of calcium carbonate owe their formation to the reversal of this dissolution process, the precipitation. Since the solubility of calcium carbonate is proportional to the amount of dissolved carbon dioxide and thus the content of carbonic acid, it is determined by the same factors that also determine the concentration of carbon dioxide (measured as partial pressure ). The complicated mechanism that is visible in some watercourses, such as the limestone terraces in Pamukkale (Turkey), is described by the lime-carbonic acid balance . The solubility of carbon dioxide increases with increasing pressure and decreasing temperature. For example, when water flows through the fine channels ( diaklases ) of limestone or volcanic rock containing calcium, it is under pressure and can therefore dissolve much more calcium carbonate. If this water then reaches the open air, the water pressure and temperature adjust to the atmospheric pressure and ambient temperature. The carbon dioxide escapes, the calcium carbonate precipitates and forms the typical calcium deposits. In this way, among other things, travertines (term, which, despite differences, is often used synonymously for limestone sinter or tufa , depending on the region and language ), more or less porous limestone rocks. Similar processes (pressure changes) take place on rapids and waterfalls of rivers, with travertines also forming. In addition to the carbon dioxide content, the amount of water present also determines the balance between dissolved and undissolved calcium carbonate. If the water content drops, a supersaturated solution is created from which calcium carbonate then precipitates. This can occur naturally through evaporation or vaporization or through freezing out. The best-known example of the precipitation of calcium carbonates through evaporation is the formation of stalactites in the karst caves of limestone: the stalactites and stalagmites . The shift in the solubility equilibrium (loss of calcium carbonate due to an increase in temperature) is also responsible for the formation of scale in boilers, on flow heaters and similar devices. Dissolution processes as the formation of structures can also be found in nature, for example in the form of styolites (protrusions a few millimeters to centimeters long or pillars that appear inside limestone banks as irregular, ribbed planes).
Similar processes can be observed in the deep sea. The calcite and aragonite compensation depth describes the depth in the sea below which the calcite and aragonite completely dissolve. The cause is the increase in the carbon dioxide concentration in the water with increasing sea depth.
Calcium carbonate is also dissolved by other acidic constituents of the air and by nitrification in the soil. Sulfur oxides contained in the air form sulfuric acid (H 2 SO 4 ) in an aqueous environment . This converts lime into calcium sulfate (CaSO 4 ) or gypsum (CaSO 4 · 2 H 2 O). Calcium sulphate is also slightly water-soluble at around 2 g / l, but it is more soluble than calcium carbonate, which leads to lime surfaces being slowly washed out by moisture.
use
Natural calcium carbonate (limestone) is used in large quantities as a raw material for the building materials industry, as an additive in the steel industry , as a mineral fertilizer , as fodder lime and as a mineral filler in various industrial applications (e.g. in paper , paints , varnishes , Plasters , plastics and backing of carpets ). In total, over five billion tons of limestone are mined every year.
Building material
The main area of application is the production of cement ( calcium silicate , calcium aluminate ) and quicklime .
Burning lime produces quicklime. From this, hydrated lime (calcium hydroxide Ca (OH) 2 , slaked lime ) is produced by extinguishing with water . It reacts with the carbon dioxide in the air to form lime and closes the technical lime cycle . Hydrated lime and lime are suitable as plaster or wall coatings such as Tadelakt . The first discoverers of this phenomenon were the Romans, who operated lime kilns on a large scale.
filler
Calcium carbonate is the most important filler by volume sold worldwide. Although more than five percent of the earth's crust consists of calcium carbonate rocks, only a few deposits are suitable for the extraction of fillers, which should be as white as possible. The largest industrial user of white calcium carbonates is the paper industry with an amount of over 10 million tons (worldwide) per year, followed by the plastics and building materials industry (plasters and paints) with a total of another 15 million tons per year. For use in the paper industry, especially as a coating color , deposits in France, Italy, Germany, Norway and Austria in particular are being mined in Europe, whereby the mineral is crushed by wet grinding and sold as a slurry (sometimes by tanker).
Agriculture
In the soil structure, the calcium-magnesium carbonate content influences the pH value and thus the structural properties, the biotic activity as well as the storage capacity and conversion of the nutrients in the soil. This makes the pH value the most important parameter for the cultivation of all soil types. A sufficient supply of lime with “free” calcium carbonate that is not bound to soil particles plays an important role. By liming attempting a soil acidification counteract and maintain soil fertility or improve. Calcium carbonate has been used to improve soil fertility in agriculture for centuries. The calcium carbonate content in the soil is determined by adding 10% hydrochloric acid and evaluating the reaction that occurs, such as foam formation.
Other areas of application
Calcium carbonate used to be used as blackboard chalk , especially in France as so-called champagne chalk, which consists of chalk rock, a chemically very pure calcium carbonate. Around 55 percent of the chalk sold in Germany today consists of gypsum (calcium sulfate).
Calcium carbonate is approved as a food additive and color (E 170) and is often used, for example, in the baking of bread rolls . For other applications, calcium carbonate is broken up and / or ground and is sold in pieces or as flour. It is contained in table salt as a trickle aid .
For some applications, natural calcium carbonates are not optimal, so synthetic calcium carbonates are used here. With the name Hydro-Calcit , synthetic calcium carbonate is used in water technology to deacidify water with " aggressive carbonic acid ". This process is still one of the oldest deacidification processes.
Together with magnesium carbonate , calcium carbonate is used in drugs for gastric acid regulation ( antacids ).
See also
- Lime soap
- Scale
- Stalactite
- Technical lime cycle , with the cycle materials calcium carbonate (this article), calcium oxide and calcium hydroxide
- Lime scale
literature
- Temple C. Patton: Pigment Handbook . Pp. 109 to 128, John Wiley & Sons, ISBN 0-471-67123-1 .
- Lothar Göttsching and Casimir Katz (eds.): Paper dictionary . Gernsbach 1999, ISBN 3-88640-080-8 .
- Wolfgang Tegethoff: Calcium carbonate . ISBN 3-7643-6424-6 .
Individual evidence
- ↑ Entry on E 170: Calcium carbonate in the European database for food additives, accessed on June 16, 2020.
- ↑ Hans-Joachim Rose: The kitchen Bible. Encyclopedia of Culinary Studies. Page 161.
- ↑ Entry on CALCIUM CARBONATE in the CosIng database of the EU Commission, accessed on January 16, 2020.
- ↑ a b c d e f g h Entry on calcium carbonate in the GESTIS substance database of the IFA , accessed on December 16, 2019(JavaScript required) .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 471-34-1 or calcium carbonate ), accessed on November 2, 2015.
- ↑ Data sheet calcium carbonate (PDF) from Merck , accessed on August 5, 2008.
- ↑ a b David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-19.
- ↑ a b c d e f g h Wolfgang F. Tegethoff: Calcium carbonate From the Cretaceous into the 21st century . Springer-Verlag, 2013, ISBN 978-3-0348-8259-0 , pp. 3 ( limited preview in Google Book Search).
- ↑ a b c Holger Nebel, Dissertation, Controlled Precipitation of CaCO 3 in a Modular Crystallization Reactor , 2008, urn : nbn: de: hbz: 465-20081222-080244-7
- ↑ a b c d e f g h i Markus Neumann: Synthesis and characterization of calcium carbonate phases and calcium phosphate-based bone substitute materials , dissertation, University of Duisburg-Essen, accessed on January 1, 2016
- ↑ a b Julyan HE Cartwright, Antonio G. Checa, Julian D. Gale, Denis Gebauer, C. Ignacio Sainz-Díaz: The polyamorphism of calcium carbonate and its importance for biomineralization: How many amorphous calcium carbonate phases are there ?. In: Angewandte Chemie. 124, 2012, p. 12126, doi: 10.1002 / anie.201203125 .
- ↑ Wissenschaft-Online-Lexika: Entry on bones in the compact lexicon of biology. Retrieved August 3, 2011.
- ↑ Ingrid Schönfelder, Peter Schönfelder: The cosmos of medicinal plants guide . Franckh-Kosmos Verlags- & Company KG, 2019, ISBN 978-3-440-16504-1 , p. 366 ( books.google.de ).
- ↑ WV Boynton, DW Ming, SP Kounaves, SM Young, RE Arvidson, MH Hecht, J. Hoffman, PB Niles, DK Hamara, RC Quinn, PH Smith, B. Sutter, DC Catling, RV Morris: Evidence for calcium carbonate at the Mars Phoenix landing site. In: Science. 325, 2009, pp. 61-64, PMID 19574384 .
- ↑ Market study fillers . Ceresana Research 2007.
- ↑ David L. Rowell: Soil science investigation methods and their applications . Springer-Verlag, 2013, ISBN 978-3-642-59093-1 , pp. 21 ( limited preview in Google Book search).
- ↑ Compakt Handbuch Chemie 1993, ISBN 3-8174-3560-6 , p. 387.
- ↑ Johann Mutschmann, Fritz Stimmelmayr: Paperback water supply . Springer-Verlag, 2007, ISBN 978-3-8348-9079-5 , p. 263 ( limited preview in Google Book search).










![{\ displaystyle \ mathrm {CaCO_ {3} +2 \ CH_ {3} COOH \ longrightarrow Ca [CH_ {3} COO] _ {2} + CO_ {2} + H_ {2} O}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/79521fe3b58cf6f8595877b471cbe706b663c9ca)

