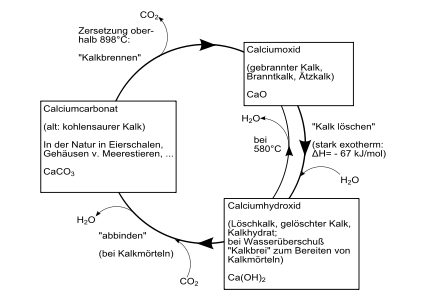
Technical lime cycle
The technical lime cycle is the technical conversion of natural limestone in three steps.
- Burn
- First calcium carbonate , the main constituent of limestone, is strongly heated (burned), carbon dioxide escapes and calcium oxide , quicklime, is formed.
- Clear
- If quicklime is mixed with water , calcium hydroxide , slaked lime, is formed.
- Setting (carbonation)
- Slaked lime reacts back to calcium carbonate, i.e. limestone, by releasing water and absorbing carbon dioxide.
Burning the lime

Calcium carbonate is a simple chemical compound with the empirical formula CaCO 3 . This mineral is found in nature, in addition to egg and mussel shells , lime sponges and corals , especially as limestone , which sometimes occurs over a large area . Typical limestones are chalk , marble , Dachstein limestone , shell limestone or travertine .
After the raw material has been extracted in the lime works , the first transformation step takes place in a lime kiln , the lime burning. From a temperature of around 1000 ° C, calcareous rock is deacidified, that is, carbon dioxide CO 2 is expelled, quick lime is formed , chemically calcium oxide CaO.
- Calcium carbonate reacts to calcium oxide and carbon dioxide when heated.
This process is eponymous for comparable processes: calcination .
When using relatively pure limestone, the white lime (fatty lime) with 90–95% CaO is produced. Otherwise one speaks of lean lime . Magnesium-containing limes with higher proportions of white magnesia (MgO) result magnesian , Magnesiakalk . Silica limes such as coral limestone or shell limestone result in limes in technical proximity to cement , both of which are harder and significantly more water-resistant building materials. Limes of inferior quality are produced when using sand-lime bricks that contain clayey components (mainly magnesium, aluminum , silicon). Higher proportions of organic components ( carbon ) remaining through the raw material or processing result in gray or black lime . Limes made from dolomite are an exception, although they contain magnesium, but due to their crystal structure correspond to high-purity white lime of comparable quality. The behavior of limes becomes even more complex when there are proportions of salts in the rock, especially rock salt (which is used in materials that set against frost).
Burning lime with fuels containing sulfur is unfavorable - the lime then partially "sulphurizes" into gypsum . A large number of solid (coal, coke, bone meal ), liquid (heating oil, solvent waste, sewage sludge) and gaseous substances (natural gas, lean gas ) are used as fuel . Rotary kilns and shaft furnaces are used. Solid fuels are added to the lime before the shaft furnace is charged.
Extinguishing the lime
The second step is usually carried out in the lime works, but can also be carried out directly by the consumer. If quick lime is mixed with water , slaked lime, chemically calcium hydroxide Ca (OH) 2 , is created with an increase in volume and strong heat development .
- Calcium oxide and water react to form calcium hydroxide.
Depending on the amount of water added, one speaks of sump lime , lime paint or lime milk . All of these shapes are used as a white paint for lime walls and as a binder for lime mortar or hydraulic mortar .
| safety instructions | ||||||||
|---|---|---|---|---|---|---|---|---|
| CAS number |
1305-78-8 |
|||||||
|
||||||||
An intermediate stage is the incompletely slaked lime , which results in a dry powder that is nevertheless capable of setting and is sold under the name hydrated lime . This forms the basis of all ready-to-use lime mortars and plasters and mixing paints that are sold in bags. Natural occurrences of hydrated lime with free silica are called pozzolans ( trass ) .
Slaked lime (calcium hydroxide) is a highly caustic , alkaline substance; Contact with the eyes can lead to blindness , inhalation of hydrated lime dusts can cause breathing problems, and unprotected skin is also attacked. Only the hardened lime, like limestone, is harmless in this regard.
Setting of the lime
In the air , slaked lime binds back to calcium carbonate with the help of carbon dioxide CO 2 , which closes the cycle. The process of setting, by the low CO 2 content of the air, the material moist and the resulting sintered layer last for years.
-
-
- Water and carbon dioxide react to form carbonic acid. Calcium hydroxide and carbonic acid react to form calcium carbonate and water.
With their high carbon content, gray and black limes carbonate significantly faster. Hydraulic limes (the above-mentioned pozzolans, cement-like limes, as well as limes enriched with porous components that store air or absorb water) also bind in a moist environment, some even under water.
If you add sand to the building lime (see aggregate ), you get lime mortar , one of the oldest building materials . The slaked lime binds between the grains of sand and strengthens the mass, while the cheap sand provides the necessary compressive strength and keeps lime consumption low. Ideally, the sand is also rich in lime, then the connection is not only mechanical, but the lime crystallizes directly on the surface of the aggregate.
With the technical names of the materials, the lime cycle is shown in the following form:
See also
- Carbonate-silicate cycle , the “natural” limestone cycle that mixes with that of the silicate rocks
- Fresco , the technique of wall painting that takes place directly within the lime cycle
literature
- Rudolf Biehler: Lime. Script at the Philipps University of Marburg, June 25, 1998 ( web document , pdf)
- Eberhart Schiele, Leo. W. Berens: Kalk. Manufacture - Properties - Use. Verlag Stahleisen, Düsseldorf 1972, ISBN 3-514-00115-4 .
- Hartmut Kainer: Coupling of heat and material exchange with chemical kinetics in the decomposition of natural carbonates. Dissertation. Clausthal University of Technology, 1982.
- Lime paperback. Bundesverlag der deutschen Kalkindustrie e. V., 2001.
Web links
- Bibliography (pdf) , Chair of Mineral Engineering, Montanuniversität Leoben (62 kB)
Individual evidence
- ↑ Kurt Wehlte : Materials and Techniques of Painting , chap. Fresco painting, materials and working methods. P. 276ff.
- ↑ https://www.atec-ltd.com/de/produkte-prozess/produkte/flexiflame-rotary-kiln-burner.html Burner for rotary kiln
- ↑ https://www.kalk.de/rohstoff/gewinnung/brennen/ Brennen von Kalk, publication of the Bundesverband der Deutschen Kalkindustrie e. V., accessed on Feb. 4, 2019
- ↑ a b Entry on calcium oxide in the GESTIS substance database of the IFA , accessed on July 29, 2017(JavaScript required) .