aluminum
| properties | |||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General | |||||||||||||||||||||||||||||||||||||
| Name , symbol , atomic number | Aluminum, Al, 13 | ||||||||||||||||||||||||||||||||||||
| Element category | Metals | ||||||||||||||||||||||||||||||||||||
| Group , period , block | 13 , 3 , p | ||||||||||||||||||||||||||||||||||||
| Appearance | silvery | ||||||||||||||||||||||||||||||||||||
| CAS number | 7429-90-5 | ||||||||||||||||||||||||||||||||||||
| EC number | 231-072-3 | ||||||||||||||||||||||||||||||||||||
| ECHA InfoCard | 100.028.248 | ||||||||||||||||||||||||||||||||||||
| Mass fraction of the earth's envelope | 7.57% | ||||||||||||||||||||||||||||||||||||
| Atomic | |||||||||||||||||||||||||||||||||||||
| Atomic mass | 26.9815385 (7) and | ||||||||||||||||||||||||||||||||||||
| Atomic radius (calculated) | 125 (118) pm | ||||||||||||||||||||||||||||||||||||
| Covalent radius | 121 pm | ||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 184 pm | ||||||||||||||||||||||||||||||||||||
| Electron configuration | [ Ne ] 3 s 2 3 p 1 | ||||||||||||||||||||||||||||||||||||
| 1. Ionization energy | 5.985 769 (3) eV ≈ 577.54 kJ / mol | ||||||||||||||||||||||||||||||||||||
| 2. Ionization energy | 18th.82855 (5) eV ≈ 1 816.68 kJ / mol | ||||||||||||||||||||||||||||||||||||
| 3. Ionization energy | 28.447 642 (25) eV ≈ 2 744.78 kJ / mol | ||||||||||||||||||||||||||||||||||||
| 4. Ionization energy | 119.9924 (19) eV ≈ 11 577.5 kJ / mol | ||||||||||||||||||||||||||||||||||||
| 5. Ionization energy | 153.8252 (25) eV ≈ 14 841.9 kJ / mol | ||||||||||||||||||||||||||||||||||||
| Physically | |||||||||||||||||||||||||||||||||||||
| Physical state | firmly | ||||||||||||||||||||||||||||||||||||
| Crystal structure | face-centered cubic | ||||||||||||||||||||||||||||||||||||
| density | 2.6989 g / cm 3 (20 ° C ) | ||||||||||||||||||||||||||||||||||||
| Mohs hardness | 2.75 | ||||||||||||||||||||||||||||||||||||
| magnetism | paramagnetic ( Χ m = 2.1 10 −5 ) | ||||||||||||||||||||||||||||||||||||
| Melting point | 933.35 K (660.2 ° C) | ||||||||||||||||||||||||||||||||||||
| boiling point | 2743 K (2470 ° C) | ||||||||||||||||||||||||||||||||||||
| Molar volume | 10.00 10 −6 m 3 mol −1 | ||||||||||||||||||||||||||||||||||||
| Heat of evaporation | 284 kJ / mol | ||||||||||||||||||||||||||||||||||||
| Heat of fusion | 10.7 kJ mol −1 | ||||||||||||||||||||||||||||||||||||
| Speed of sound | 6250-6500 ( longitudinal wave ) m / s; 3100 ( shear wave ) ms −1 at 293.15 K |
||||||||||||||||||||||||||||||||||||
| Specific heat capacity | 897 J kg −1 K −1 | ||||||||||||||||||||||||||||||||||||
| Work function | 4.06-4.26 eV | ||||||||||||||||||||||||||||||||||||
| Electric conductivity | 37.7 · 10 6 A · V −1 · m −1 | ||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 235 W m −1 K −1 | ||||||||||||||||||||||||||||||||||||
| Mechanically | |||||||||||||||||||||||||||||||||||||
| Modulus of elasticity | 60 to 78 kN / mm² | ||||||||||||||||||||||||||||||||||||
| Poisson's number | 0.34 | ||||||||||||||||||||||||||||||||||||
| Chemically | |||||||||||||||||||||||||||||||||||||
| Oxidation states | 1, 2, 3 | ||||||||||||||||||||||||||||||||||||
| Normal potential | −1.676 V (Al 3+ + 3 e - → Al) | ||||||||||||||||||||||||||||||||||||
| Electronegativity | 1.61 ( Pauling scale ) | ||||||||||||||||||||||||||||||||||||
| Isotopes | |||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
| For other isotopes see list of isotopes | |||||||||||||||||||||||||||||||||||||
| NMR properties | |||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
| safety instructions | |||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
|
As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . |
|||||||||||||||||||||||||||||||||||||
Aluminum (often also aluminum in the Anglo-American language area ) is a chemical element with the element symbol Al and the atomic number 13. In the periodic table , aluminum belongs to the third main group and to the 13th IUPAC group , the boron group , which was previously referred to as the group of earth metals . There are numerous aluminum compounds .
Aluminum is a silvery-white light metal . In the earth sheath , it is, by oxygen and silicon , the third most abundant element and in the Earth's crust The most common metal.
In materials engineering , "aluminum" means all materials based on the element aluminum. This includes pure aluminum (at least 99.0% Al), pure aluminum (at least 99.7% Al) and especially the aluminum alloys , which have strengths that are comparable to steel, with only a third of its density.
Aluminum, which occurs almost exclusively in the form of chemical compounds, was discovered in the early 19th century. Industrial mass production began in the early 20th century.
The mining takes place in aluminum smelters starting from the mineral bauxite first in the Bayer process , with which aluminum oxide is obtained, and then in the Hall-Héroult process of a fused- salt electrolysis , in which aluminum is obtained. In 2016, 115 million tons of aluminum oxide (Al 2 O 3 ) were produced worldwide . 54.6 million tons of primary aluminum were extracted from this.
The metal is very ignoble and reacts in freshly cut areas at room temperature with air and water to form aluminum oxide . However, this immediately forms a thin layer impermeable to air and water ( passivation ) and thus protects the aluminum from corrosion . Pure aluminum has poor strength; it is significantly higher for alloys. The electrical and thermal conductivity is high, which is why aluminum is used for lightweight cables and heat exchangers.
One of the most famous products is aluminum foil . Others include components in vehicles and machines, electrical cables, pipes, boxes and household items. The aluminum recycling achieved world rates of about 40%.
history
In 1782 Lavoisier was the first to suspect that the alum earth ( alumina , derived from the Latin alumen 'alum'), obtained by Marggraf in 1754 from an alum solution , was the oxide of a previously unknown element. Its representation finally succeeded in 1825 the Dane Hans Christian Oersted by reaction of aluminum chloride (AlCl 3 ) with potassium amalgam , wherein potassium as reducing agent was used:
Davy , who had also tried to represent the new element for a long time, introduced the name variants alumium , aluminum and aluminum from 1807 , of which the last two continue to exist side by side in English.
In 1827 Friedrich Wöhler succeeded in obtaining purer aluminum using the same method as Ørsted, but using metallic potassium as a reducing agent. Henri Étienne Sainte-Claire Deville refined the Wöhler process in 1846 and published it in a book in 1859. This improved process increased the yield from aluminum extraction, and as a result, the price of aluminum, which had previously been higher than that of gold, fell to a tenth within ten years.
In 1886, Charles Martin Hall and Paul Héroult independently developed the electrolysis process named after them for the production of aluminum: the Hall-Héroult process . In 1889, Carl Josef Bayer developed the Bayer process, named after him, for isolating pure aluminum oxide from bauxites. Aluminum is still produced on an industrial scale according to this principle.
At the end of the 19th century the metal was so respected that metal ships made from it were baptized with the name Aluminia .
Occurrence
With a share of 7.57 percent by weight, aluminum is the third most common element in the earth's crust after oxygen and silicon and is therefore the most common metal . However, due to its ignoble character, it occurs almost exclusively in bound form. The largest amount is chemically bound in the form of aluminosilicates , in which it takes the position of silicon in oxygen tetrahedra in the crystal structure . These silicates are components of clay , gneiss and granite , for example .
More rarely, aluminum oxide is found in the form of the mineral corundum and its varieties ruby (red) and sapphire (colorless, different colors) . The colors of these crystals are based on the addition of other metal oxides. At almost 53 percent, corundum has the highest aluminum content in a compound. The even rarer minerals akdalaite (about 51 percent) and diaoyudaoite (about 50 percent) have a similarly high aluminum content . A total of 1156 aluminum-containing minerals are known to date (as of 2017).
The only economically important starting material for aluminum production is bauxite . Deposits are located in southern France ( Les Baux ), Guinea, Bosnia and Herzegovina, Hungary, Russia, India, Jamaica, Australia, Brazil and the United States. Bauxite contains about 60 percent aluminum hydroxide (Al (OH) 3 and AlO (OH)), about 30 percent iron oxide (Fe 2 O 3 ) and silicon dioxide (SiO 2 ).
In production, a distinction is made between primary aluminum , also known as primary aluminum , which is obtained from bauxite, and secondary aluminum from aluminum scrap . The recycling requires only about 5 percent of the energy of the primary production.
Aluminum as a mineral
As a result of passivation, aluminum is very rarely found in nature in an elementary ( native ) form. Aluminum was first discovered in 1978 by BV Oleinikov, AV Okrugin, NV Leskova in mineral samples from the Billeekh Intrusion and the Dyke OB-255 in the Republic of Sakha (Yakutia) in the Russian Federal District Far East. A total of around 20 sites (as of 2019) for solid aluminum are known worldwide, including in Azerbaijan , Bulgaria , the People's Republic of China ( Guangdong , Guizhou , Jiangsu and Tibet ) and in Venezuela . In addition, solid aluminum could be detected in rock samples from the moon , which the probe of the Luna 20 mission brought from the Apollonius crater .
Due to its extreme rarity, solid aluminum is of no importance as a source of raw materials, but as a genuine element, aluminum is recognized by the International Mineralogical Association (IMA) as an independent mineral (internal entry number of IMA: 1980-085a ). According to the systematics of minerals according to Strunz (9th edition) , aluminum is classified under the system number 1.AA.05 (elements - metals and intermetallic compounds - copper cupalite family - copper group). However , aluminum is not yet listed in the outdated 8th edition of Strunz's mineral classification. Only in the "Lapis mineral directory", which was last updated in 2018, which is still based on this form of system numbering out of consideration for private collectors and institutional collections, the mineral was given the system and mineral number. I / A.3-05 . The systematics of minerals according to Dana , which is mainly used in English-speaking countries , lists the element mineral under the system no. 01/01/01/05.
In nature, solid aluminum usually occurs in the form of granular mineral aggregates and micro nuggets , but in rare cases it can also develop tabular crystals up to a millimeter in size. Fresh mineral samples have a shiny metallic , silvery white color. In the air, the surfaces darken due to oxidation and appear gray. Aluminum leaves a dark gray line on the marking board .
Depending on where it was found, aluminum often contains foreign admixtures of other metals (Cu, Zn, Sn, Pb, Cd, Fe, Sb) or occurs ingrown or microcrystalline intergrown with hematite , ilmenite , magnetite , moissanite and pyrite or jarosite .
Type material , i.e. mineral samples from the type locality of the mineral, is kept in the Geological Museum of the Academy of Sciences in Yakutsk in the Russian republic of Sakha (Yakutia).
Extraction
Primary aluminum (made from minerals)
Approx. 2/3 of the European aluminum demand is covered by primary aluminum. Primary aluminum is produced electrolytically from an aluminum oxide melt. Since this is difficult to isolate from the aluminosilicates that are ubiquitous on earth, industrial production takes place from the relatively rare, low-silicate bauxite . There have been proposals for a long time to obtain pure aluminum oxide from silicates, but their use is not economically feasible.
The aluminum oxide / hydroxide mixture contained in the ore is first digested with caustic soda ( Bayer process , tubular reactor or autoclave digestion) in order to free it from foreign components such as iron and silicon oxide, and is then mainly in fluidized bed systems (but also in Rotary kilns ) burned to aluminum oxide (Al 2 O 3 ).
The dry digestion ( Deville method ), on the other hand, is no longer relevant. Here, finely ground, unpurified bauxite was calcined together with soda and coke in rotary kilns at around 1200 ° C and the resulting sodium aluminate was then dissolved with sodium hydroxide solution.
The preparation of the metal to occur in aluminum smelters by fused-salt electrolysis of aluminum oxide after the cryolite-alumina procedure ( Hall-Héroult process ). To lower the melting point, the aluminum oxide is melted together with cryolite ( eutectic at 963 ° C). During electrolysis , aluminum is produced at the cathode, which forms the bottom of the vessel, and oxygen is produced at the anode , which reacts with the graphite (carbon) of the anode to form carbon dioxide and carbon monoxide . The graphite blocks that make up the anode slowly burn off and are replaced from time to time. The graphite cathode (vessel bottom) is inert to aluminum . The liquid aluminum that collects on the floor is sucked off with a suction pipe.

Due to the high binding energy due to the trivalent nature of aluminum and the low atomic mass, the process is quite energy-consuming. 12.9 to 17.7 kilowatt hours of electrical energy must be used per kilogram of raw aluminum produced . A reduction in electricity demand is only possible to a small extent, because the potential for energy optimization has largely been tapped. Aluminum production is therefore only economical if cheap electrical energy is available, for example next to hydropower plants, such as in Rheinfelden or (formerly) in Ranshofen not far from the Inn.
| rank | country | production | Reserves |
|---|---|---|---|
| 1 |
|
75,000 | 6,000,000 |
| 2 |
|
70,000 | 1,000,000 |
| 3 |
|
50,000 | 7,400,000 |
| 4th |
|
27,000 | 2,600,000 |
| 5 |
|
24,000 | 660,000 |
| 6th |
|
10,000 | 2,000,000 |
| 7th |
|
7,100 | 1,200,000 |
| 8th |
|
5,500 | 500,000 |
| 9 |
|
2,500 | 3,700,000 |
| 10 |
|
2,000 | 110,000 |
The following table shows the aluminum production and the maximum possible production output of the steelworks by country.
| rank | country | production | capacity |
|---|---|---|---|
| 1 |
|
33,000 | 47,800 |
| 2 |
|
3,700 | 4,060 |
| 3 |
|
3,700 | 3,900 |
| 4th |
|
2,900 | 3,270 |
| 5 |
|
2,600 | 2,600 |
| 6th |
|
1,600 | 1,720 |
| 7th |
|
1,300 | 1,430 |
| 8th |
|
1,000 | 1,050 |
| 9 |
|
890 | 1,790 |
| 10 |
|
870 | 870 |
Secondary aluminum (produced by aluminum recycling)
To recycle aluminum, aluminum scrap and “ dross ” are melted down in drum furnaces. "Scabies" is a waste product from the processing of aluminum and the production of secondary aluminum. Dross is a mixture of aluminum metal and fine-grain oxide particles and is formed when aluminum is melted at 800 ° C from the aluminum oxide of normal aluminum corrosion and as an oxidation product (oxide skin) when liquid aluminum comes into contact with atmospheric oxygen. So that no aluminum oxide particles from entering the casting in aluminum casting, the itch is scratching devices from the surface of the metal bath skimmed off.
To prevent the formation of dross, the surface of the melt is covered with halide salts (around two thirds NaCl, one third KCl and small amounts of calcium fluoride CaF 2 ) (see aluminum recycling ). The by-product of this is salt slag, which still contains around 10 percent aluminum, which, when processed accordingly, serves as a raw material for mineral glass fibers.
However, the production of secondary aluminum has been criticized for the fact that every tonne produces 300 to 500 kilograms of salt slag contaminated with dioxins and metals; however, their possible recycling is state of the art.
properties
Physical Properties
Microstructure
Aluminum solidifies exclusively in a face-centered cubic space lattice in space group Fm 3 m (space group no. 225) . The lattice parameter for pure aluminum is 0.4049 nm (corresponds to 4.05 Å ) with 4 formula units per unit cell .
Vacancies occur with a density of 1.3 × 10 −4 at 500 ° C, at room temperature it is only 10 −12 . By quenching larger spaces densities can occur at room temperature, which is for some of the properties of aluminum materials is important because the vacancies are the diffusion favor. By forming at room temperature, the density of vacancies can 10 -4 be increased. The dislocation density is 10 −7 , a range typical for metals, and leads to good formability of aluminum. Stacking faults could not be detected in aluminum, which is explained by the high stacking fault energy of 103 to 200 (10 −7 J / cm²). This means that the strength increase in cold rolling and forging is only slight and some aluminum materials even tend to soften afterwards.
density
With a density of 2.6989 g / cm³ (about a third of steel), aluminum is a typical light metal , which makes it an interesting material for lightweight construction . The density of the alloys usually only deviates by around +3% to −2%. Special alloys with lithium have a 15% lower density. Aluminum is therefore one of the lightest materials, surpassed only by magnesium .
Mechanical properties
Aluminum is a relatively soft and tough metal. The tensile strength of absolutely pure aluminum is 45 N / mm², the yield point is 17 N / mm² and the elongation at break is 60%, while the tensile strength of commercially pure aluminum is 90 N / mm², the yield point is 34 N / mm² and the Elongation at break at 45%. In contrast, the tensile strength of its alloys is up to 710 N / mm² (alloy 7068). Its modulus of elasticity is around 70 GPa, a value that is often given. A value of 66.6 GPa is given for pure aluminum, but the values vary from 60 to 78 GPa. The G-module is 25.0 kN / mm², the Poisson's ratio (Poisson's ratio) at 0.35.
Thermal properties
The melting temperature is 660.2 ° C and the boiling temperature is 2470 ° C. The melting temperature is significantly lower than that of copper (1084.6 ° C), cast iron (1147 ° C) and iron (1538 ° C), which makes aluminum a good cast material .
At a transition temperature of 1.2 K, pure aluminum becomes superconducting .
The thermal conductivity is relatively high at 235 W / (K m). The thermal conductivity of copper is about twice as high, but the density is about four times greater, which is why aluminum is used for heat exchangers in vehicles. The coefficient of thermal expansion is quite high at 23.1 µm · m −1 · K −1 due to the very low melting point .
The shrinkage , i.e. the decrease in volume during solidification, is 7.1%.
Electrical Properties
Since thermal and electrical conductivity are dominated by the same mechanisms in metals, aluminum is also a very good electrical conductor. In the ranking of the elements with the greatest specific conductivity , aluminum ranks fourth behind silver, copper and gold, as is the case with thermal conductivity. Due to the combination of high specific conductance, low density, high availability and (compared to other materials) low costs, aluminum has become the most important conductor material alongside copper in electrical engineering - especially in power engineering where large conductor cross-sections are required.
Magnetic properties
Aluminum is paramagnetic , so it is attracted by magnets, but the effect is very weak. The magnetic susceptibility at room temperature is 0.62 × 10 −9 m³ / kg, which means that aluminum is practically non-magnetic.
Chemical properties
The pure light metal aluminum has a dull, silver-gray appearance due to a thin oxide layer that forms very quickly in the air . This passivating oxide layer makes pure aluminum very resistant to corrosion at pH values of 4 to 9 , it reaches a thickness of about 0.05 µm.
This oxide layer protects against further oxidation, but is a hindrance when making electrical contact and when soldering . It can be strengthened by electrical oxidation ( anodizing ) or chemically.
The oxide layer can be dissolved by means of complex formation reactions. In a neutral chloride solution, aluminum forms an extremely stable and water-soluble neutral complex. The following equation illustrates the process:
This is preferably done in places where the oxide layer of the aluminum is already damaged. There is pitting corrosion due to the formation of holes . If the chloride solution can then reach the free metal surface, other reactions take place. Aluminum atoms can be oxidized with complexation:
If ions of noble metals are present in the solution, they are reduced and deposited on the aluminum. The reduction of silver ions, which are present as silver sulfide on the surface of tarnished silver , to silver is based on this principle .
Aluminum reacts violently with aqueous sodium hydroxide solution (NaOH) (and a little less violently with aqueous sodium carbonate solution ) to produce hydrogen . This reaction is exploited in chemical pipe cleaning agents. The reaction of aluminum with NaOH takes place in two steps: the reaction with water and the complexation of the hydroxide to sodium aluminate .
When reacting with water
First aluminum hydroxide is formed.
As a rule, the surface is then dried, during which the hydroxide is converted into the oxide:
However, this does not happen when aluminum reacts in aqueous sodium hydroxide solution .
Now the 2nd step follows, the complexation of the hydroxide to sodium aluminate:
As a result of the complexation, the gelatinous hydroxide becomes water-soluble and can be transported away from the metal surface. As a result, the aluminum surface is no longer protected from further attack by the water and step 1 runs again.
As with the reaction of aluminum with acids, this method can produce three moles of hydrogen gas for every two moles of aluminum.
At room temperature, aluminum reacts with bromine with the appearance of flames. It should be noted that the resulting aluminum bromide reacts with water to form aluminum hydroxide and hydrobromic acid.
Aluminum forms an amalgam with mercury . When mercury comes in direct contact with aluminum, i. That is, if the aluminum oxide layer is mechanically destroyed at this point, mercury eats holes in the aluminum; Under water, aluminum oxide then grows above it in the form of a small cauliflower. For this reason, mercury is classified as a dangerous good and a “corrosive liquid” in relation to aluminum materials in aviation .
Aluminum reacts very violently with hydrochloric acid, developing hydrogen; sulfuric acid slowly dissolves it. It is passivated in nitric acid.
In powder form (particle size less than 500 µm), aluminum is very reactive due to its large surface , especially if it is not phlegmatized . Aluminum then reacts with water, releasing hydrogen to form aluminum hydroxide. The finest, non-phlegmatized aluminum powder is also known as pyro cut. Aluminum dust that has not been phlegmatized is very dangerous and spontaneously ignites upon contact with air.
Isotopes
The isotope 27 Al occurs exclusively in nature ; Aluminum is one of the pure elements . This isotope, which is stable and contains 14 neutrons and 13 protons in the nucleus , does not absorb neutrons, which is why aluminum is used in nuclear reactors . All other isotopes are produced artificially and are radioactive . The most stable of these isotopes is 26 Al with a half-life of one million years. By electron or beta decay it created 26 Mg, by capturing a neutron and subsequent gamma decay 27 Al. The isotopes 24 Al to 29 Al (except for 26 Al and 27 Al) have half-lives between a few seconds and a few hundred seconds. 23 Al decays with a half-life of only 0.13 seconds.
Aluminum alloys
Aluminum alloys are alloys that mainly consist of aluminum. For other alloys that contain aluminum, see section #Other Applications .
Aluminum can be alloyed with numerous metals to promote certain properties or to suppress other, undesirable properties. With some alloys, the formation of the protective oxide layer ( passivation ) is severely disturbed, which means that the components made from them are sometimes at risk of corrosion . Almost all high strength aluminum alloys are affected by the problem.
There are wrought aluminum alloys that are intended for further processing by rolling , forging and extrusion , and cast materials . These are used in foundries.
In general, aluminum alloys are divided into two large groups of wrought and cast alloys:
- Cast aluminum alloys. Typical cast aluminum alloys contain silicon as the main alloying element ( AlSi ), but there are also types with copper or magnesium as cast alloys.
- Wrought aluminum alloys, they have a share of about 75% and are further subdivided according to the main alloy element (s) into
- Pure aluminum with an aluminum content of 99.0% to 99.9%. They are very easy to work with, have low strength and good corrosion resistance.
- Aluminum-copper alloys (AlCu): They have medium to high strength, can be hardened, but are susceptible to corrosion and difficult to weld. They can contain magnesium or manganese additives.
- Aluminum-manganese alloys (AlMn): They have low to medium strength, are corrosion-resistant and easy to process.
- Aluminum-magnesium alloys (AlMg, without AlMgSi): They have medium strengths, cannot be hardened, are corrosion-resistant, easy to form and weld. Most types also contain manganese (AlMg (Mn)).
- Aluminum-magnesium-silicon alloys (AlMgSi): They have medium to high strengths, can be easily processed by welding and extrusion, can be hardened and are corrosion-resistant.
- Aluminum-zinc-magnesium alloys (AlZnMg): Copper-free types have medium to high strengths and are easy to weld. Types containing copper (AlZnMg (Cu)) have high strengths - in the case of 7075 over 500 MPa - cannot be processed by fusion welding , but can be machined (milling, drilling).
- Special alloys, for example aluminum-lithium alloys with a particularly low density, or free -cutting alloys that are particularly easy to machine .
A distinction is also made between naturally hard alloys - which cannot be hardened by heat treatment - and hardenable alloys:
- Typical naturally hard wrought aluminum alloys are: AlMg, AlMn, AlMgMn, AlSi
- Hardenable wrought alloys - increase in strength through precipitation hardening of alloy elements with additional aging annealing at 150 to 190 ° C. Typical hardenable wrought aluminum alloys are: AlMgSi, AlCuMg, AlZnMg. The first high-strength, hardenable aluminum alloy AlCuMg was given the trade name Duraluminium , or “Dural” for short , in 1907 .
Economical meaning
After steel, aluminum is the second most important metallic material. 115 million tons were produced worldwide in 2016.
The price of aluminum on the world market has been around $ 2,000 per ton (purity of 99.7%) since 1980. However, it is relatively volatile , falling to around $ 1,500 per ton in 2016, while it was back to close to $ 2,000 in 2017.
use
Construction material in general
Aluminum has a high specific strength . Compared to steel , components made of aluminum are about half as heavy with the same strength, but have a larger volume. Therefore, it is often in lightweight used ie where demand low mass, for example in transport contributes to lower fuel consumption, especially in the aviation and aerospace . In motor vehicle construction , it won because of this importance; In the past, the high material price, poor weldability and the problematic fatigue strength and deformation properties in the event of accidents (low energy absorption in the so-called crumple zone ) stood in the way. The hood of the Washington Monument , a 3 kg casting, was considered one of the largest aluminum workpieces until 1884. When building small and medium-sized ships and boats, the corrosion resistance of aluminum to salt water is valued. Vehicle construction (including ships, airplanes and rail vehicles) accounted for the largest share of the global use of aluminum in 2010 with around 35 percent.
In aluminum alloys , strengths are achieved that are only slightly inferior to those of steel . Therefore, the use of aluminum to reduce weight is appropriate wherever material costs play a subordinate role. Aluminum and duralumin are particularly widespread in aircraft construction and space technology. The largest part of the structure of today's commercial aircraft is aluminum sheets of various thicknesses and alloys riveted .
vehicle construction
In vehicles, their mass plays a role: the lighter the vehicle, the lower the fuel consumption. In Germany, almost 50% of aluminum is used in vehicle construction (as of 2015).
- cars
In cars aluminum materials are used for various engine components - including the engine block , the cylinder piston for the special piston alloys exist, the cylinder heads - where especially the low thermal expansion and Korrosionsanfäligkeit and high temperature strength are crucial; along with the good castability , as these components are usually cast. Other applications in vehicles are designed for housing of transmissions , as a heat shield and a heat exchanger - the last two in the form of pure aluminum. In the chassis , aluminum is used as forged parts for rear axles , axle carriers , wishbones and wheels. In the body, aluminum is used for doors, hoods , bumpers and fenders , as well as in the body shell structure.
- commercial vehicles
In commercial vehicles , aluminum is used for drop sides , tail lifts , superstructures, for load securing , compressed air tanks , fuel tanks and as underbody protection . In commercial vehicles, lightweight construction with aluminum is heavily influenced by the legal maximum load per axle: a higher payload is possible with a lower vehicle weight.
- Rail vehicles
Aluminum is also used abundantly in rail vehicles . The prerequisite for this were two other important developments: certain welding processes that are suitable for aluminum materials ( TIG welding / MIG welding ) in the 1950s and the extrusion of large profiles . The use of aluminum has changed the entire construction of rail vehicles. Constructions made of steel tubes were common up to around 1970, afterwards increasingly welded aluminum profiles.
- Planes
Already in the early stages of aviation, aluminum materials were used, in 1903 magnalium, for example, for the fittings of an airplane, which was still largely made of wood, wire and cloth. The first airworthy all-metal airplane dates from 1915, but consisted of sheet steel in a shell construction. The decisive development for the use of aluminum in aircraft construction comes from Alfred Wilm in 1906 , who found a hardenable aluminum-copper alloy in duralumin , which has very high strengths and is therefore ideally suited for lightweight construction. AlCu and AlZnMg are used for aircraft . 60% of the total mass of aircraft is due to aluminum. The connection of the workpieces stamped, cut or driven from sheet metal, milled from the solid or consisting of profiles is usually done by riveting , since the most commonly used materials are difficult to weld.
Electrical engineering
Electric lines

Aluminum is a good conductor of electricity . After silver, copper and gold, it has the fourth highest electrical conductivity of all metals. For a given electrical resistance, an aluminum conductor has a smaller mass but a larger volume than a copper conductor. This is why copper is mostly used as an electrical conductor when volume plays a dominant role, such as with the windings in transformers . Aluminum has advantages as an electrical conductor when weight plays an important role, for example in the conductors of overhead lines . In order to reduce weight, aluminum cables are used in aircraft such as the Airbus A380 .
Among other things, aluminum is processed into busbars in substations and into current-carrying cast parts . There are copper-clad aluminum cables for electrical installations ; the copper coating is used to improve contact. In these areas of application, raw material prices are primarily decisive, since aluminum is cheaper than copper. On the other hand, it is unsuitable for overhead lines in electric railways due to its poor contact and sliding properties; in this area, copper is primarily used despite its higher weight.
Aluminum is problematic when contacted under pressure because it has a tendency to creep . In addition, it becomes coated with an oxide layer in air. After long periods of storage or contact with water, this insulating layer is so thick that it must be removed before contact is made. Bimetal corrosion occurs particularly in contact with copper . In the case of unsuitable contacts in terminals , aluminum conductors can result in failures and cable fires due to loosening contacts. Crimp connections with suitable sleeves and tools are safe, however. As an intermediate layer between copper and aluminum, connecting pieces made of Cupal can avoid contact problems.
The slight decrease in the specific electrical conductivity of aluminum when alloy components are added should be emphasized, whereas copper shows a significant reduction in conductivity when contaminated.
electronics
The electronics industry uses aluminum because of its good processability and good electrical and thermal conductivity .
Until the 2000s, aluminum was the only conductor material used in integrated circuits . Until the 1980s it was used as a material for the control electrode (gate) of field effect transistors with a metal-insulator-semiconductor structure ( MOSFET or MOS-FET). In addition to the low specific resistance, the good adhesion to and low diffusion in silicon oxides (insulation material between the conductor tracks) as well as the easy structuring with the help of dry etching are decisive for the use. Since the beginning of the 2000s, however, aluminum has increasingly been replaced by copper as a conductor track material, even if more complex structuring processes (cf. Damascene and dual Damascene processes ) and diffusion barriers are necessary. The higher manufacturing effort is due to the lower specific resistance, which increases significantly earlier in the case of small structures in aluminum and outweighs other properties (e.g. electromigration behavior ) and the aluminum processes were able to meet the increased requirements ( clock frequency , power loss ) with high Frequencies are no longer sufficient for working circuits.
However, aluminum is still used in microelectronic products. Because of its good contactability with other metals, it is used in the last conductor track levels in order to establish electrical contact with the solder balls used in flip-chip assembly . The situation is similar with power semiconductors, in which all conductor track levels are usually made of aluminum. In general, and particularly in the case of power semiconductors , the material is used for bonding wires (connecting wires between the chip and the housing connection).
With the introduction of high-k + metal gate technology , aluminum has good after 25 years of abstinence also gained importance in the area of the gate and is in addition to other as the material for setting the work function used.
Packaging and container

In the packaging industry, aluminum is processed into beverage and food cans as well as aluminum foil . The property of the absolute barrier effect against oxygen, light and other environmental influences is used. The decisive factor for the use of aluminum as packaging is not its low density, but its good processability by rolling and its non-toxicity. Thin films are produced in thicknesses of six micrometers and then mostly used in composite systems, for example in Tetra Paks . Plastic films can be provided with a thin layer by vapor deposition with aluminum, which then has a high (but not complete) barrier function. The reason for this barrier effect is not the pure aluminum, but the passive layer made of boehmite . If this is damaged, gas can flow unhindered through the aluminum material. Mostly pure aluminum, AlMn (alloys with manganese) and AlMg (alloys with magnesium) are used.
Cooking pots and other kitchen utensils, such as the classic Italian espresso pot , as well as travel and military dishes are made from aluminum .
Aluminum is processed for a large number of containers and housings because it can be easily processed by forming. Objects made of aluminum are often protected from oxidation and abrasion by an anodized layer .
In 2017, packaging accounted for 17% of European aluminum use.
Optics and lighting technology
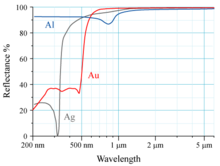
Due to its high degree of reflection, aluminum is used as a mirror coating for surface mirrors, for example in scanners , motor vehicle headlights and reflex cameras, but also in infrared measurement technology. In contrast to silver, it reflects ultraviolet radiation . Aluminum mirror layers are usually protected from corrosion and scratches by a protective layer.
Architecture and construction
Concrete production
Aluminum powder and aluminum pastes are used to manufacture aerated concrete . To use compounds such as aluminum hydroxysulphate , Aluminiumdihydroxyformiat or amorphous aluminum hydroxide as an alkali-free shotcrete accelerators .
Construction and functional materials
Aluminum is used as a construction material for load-bearing parts of buildings and as a functional material as decorative, corrosion-resistant parts. In addition to the weather resistance, the good processability is crucial, especially in the case of manual production. The construction industry is the main customer for aluminum profiles. Aluminum is mainly used for window frames , doors and elements of facades . The facade of the Imperial War Museum in Manchester is particularly well known . The aluminum-manganese alloys, which have low strength and good corrosion resistance, are mainly used . In some cases, aluminum is used for bridge construction , where otherwise steel construction predominates. Alloys with higher strength, including AlMg and AlSi, are used for structural engineering . Sheets and composite panels made of aluminum alloys achieve fire protection classes from 'non-combustible' to 'normally flammable'. A house fire develops 1000 ° C heat in a full fire, which, regardless of the fire protection class, burns holes in the aluminum alloy, which flows or drips down between 600 ° C and 660 ° C.
Other uses
In rocket technology, the fuel for solid rocket rockets consists of a maximum of 30 percent aluminum powder, which releases a lot of energy when it burns. Aluminum is used in fireworks (see also pyrotechnics ), where it creates colored effects depending on the grain size and mixture. It is also often used in bang sentences.
In aluminothermics , aluminum is used to extract other metals and semi-metals by using the aluminum to reduce the oxides. An important process in aluminothermics is the thermite reaction , in which aluminum is reacted with iron (III) oxide. This strongly exothermic reaction creates temperatures of up to 2500 ° C and liquid iron that is used for aluminothermic welding, e.g. As for joining of train tracks . Further applications of the reducing effect of aluminum are made possible for laboratory purposes by using aluminum amalgam .
Aluminum serves as a pigment for colors (silver or gold bronze). Colored anodized , it is part of many decorative materials such as tinsel, ribbon and tinsel. It is used to coat surfaces in aluminizing .
Heating elements of irons and coffee machines are pressed around with aluminum .
Before it was possible to make zinc sheet workable as so-called titanium zinc by adding titanium , aluminum sheet was used for facade and roof elements (see lightweight roof ) and gutters.
Because of its high thermal conductivity, aluminum is used as a material for extruded heat sinks and heat-dissipating base plates. Aluminum electrolytic capacitors use aluminum as the electrode material and housing material; it is also used to manufacture antennas and waveguides .
Aluminum is found in some alloys. In addition to the aluminum alloys, which mainly consist of aluminum, it also occurs in the copper alloys aluminum bronze , aluminum brass , isabelline , in roughly equal parts Al and copper in the Devardas alloy , as the main alloy element for magnesium alloys and in Alnico and Sendust , two iron alloys with special magnetic properties . Aluminum is also found in many titanium alloys , especially in Ti-6Al-4V , the type that makes up about 50% of all titanium alloys. It contains six percent aluminum by mass.
processing
During processing, a distinction is made between cast alloys and wrought alloys :
- Casting alloys are processed in foundries and poured into molds that already completely or largely correspond to the shape of the end products. This is followed by finishing by grinding. Cast alloys are often melted from scrap.
- Wrought alloys are cast into bars in the smelting works and then rolled there to produce plates, sheets, rods and foils. Individual parts are produced from thick plates and other solid raw parts by machining (milling, drilling and turning). Other solid raw parts can be processed into individual pieces by forging or into profiles by extrusion. The latter is particularly common with aluminum. Sheets are processed by punching, bending and deep drawing .
The individual parts are then connected by welding, riveting, soldering and similar processes.
to water
The casting of aluminum is known as aluminum casting . Due to its comparatively low melting point of 660 ° C ( cast iron around 1150 ° C, steel 1400 ° C to 1500 ° C) and its good castability, it is one of the materials often used in foundries . AlSi , special casting alloys with silicon, even have melting points around 577 ° C. The mass fraction of aluminum in all products produced in foundries is around 11% (cast iron 76%, cast steel 9%) and is therefore by far the most important non-ferrous metal (non-ferrous metals) in foundries , ahead of copper with 1.5%. The proportion of non-ferrous metal casting of aluminum is around 87%. In 2011, around 840,000 tons of aluminum were processed in foundries in Germany; About 76% of non-ferrous metal castings are used in the automotive industry.
The low melting point results in a low energy consumption in the melting process and a lower temperature load on the molds. Aluminum is basically suitable for all casting processes , in particular for die casting or aluminum die casting , with which parts with complex shapes can be manufactured. Special aluminum casting alloys are processed in the foundry , mostly aluminum-silicon alloys . In the smelting works, on the other hand, mostly wrought alloys are produced which are intended for further processing by rolling , forging and extrusion . In the smelting works, these are cast into bars ( ingot casting ) or round bars, which in theory can be endless ( continuous casting ). Continuous casting has been used more and more since the 1930s. There are special systems for this which can produce up to 96 round bars at the same time with casting lengths between 3 and 7 meters, sometimes up to 10 meters. The diameters are between 75 and 700 mm. Sheets are sometimes made by casting directly onto a roller that cools the melt. The raw sheet is then directly cold rolled without hot rolling , which saves costs of up to 60%.
Forming processes
About 74 percent of the aluminum is processed by forming . These include rolling, forging, extrusion and bending.
Pure and ultra-pure aluminum can be easily formed due to its low strength and solidifies during cold forming , whereby large changes in shape are possible. The solidification can be removed by recrystallization annealing . Wrought alloys with AlMg and AlMn achieve their higher strength through the alloying elements and through cold forming. The hardenable alloys AlMgSi , AlZnMg , AlCuMg and AlZnMgCu separate during forming strength-increasing stages of; they are relatively difficult to form.
- Rollers
Cast bars are often processed further by rolling , either into thick plates that are then milled into end products, into sheets that are further processed by punching and bending or into foils. The microstructure of the materials changes during rolling: Small spherical components that are often present after casting are flattened and stretched. This makes the structure finer and more even on the one hand, but also direction- dependent on the other . The capacity of an aluminum hot rolling mill is around 800,000 tons per year. Bars with a mass of up to 30 tons are processed. They have dimensions of up to 8.7 meters in length, 2.2 meters in width and 60 cm in thickness. Even larger bars can be processed technically, but the quality of the structure then decreases. After hot rolling , the material is usually about 20 to 30 mm thick. This is followed by cold rolling to the final thickness. Cold rolling mills have capacities of 300,000 to 400,000 tons per year. Composite materials can be made by roll cladding . A layer of a different material is applied to one or both sides. A layer of corrosion-resistant pure aluminum is often applied to the core material that is susceptible to corrosion.
- Extrusion
Aluminum can be extruded into complex structural profiles ; This is a great advantage in the manufacture of hollow profiles (e.g. for window frames, rods, beams), heat sink profiles or in antenna technology. Semi-finished products or components are manufactured from raw materials such as rolling ingots , sheet metal or cylinders . Aluminum alloys can be extruded much better than other materials, which is why a large part of the aluminum is processed using this process. The raw material is pressed through a hollow tool. The result is endless material that is sawn off to the desired length. Complicated cross-sections can also be produced, for example hollow profiles or those with undercuts. However, the cross section is constant over the length. With high-strength alloys, large minimum wall thicknesses are required and pressing takes a long time, which is why the medium-strength, age-hardenable alloys are preferred. The curing is usually carried out immediately afterwards. During extrusion, the material is heated to temperatures of around 450 to 500 ° C in order to increase formability, which is also used for solution annealing . Immediately after extrusion, the workpiece is strongly cooled by air or water and thus quenched , which leads to higher strengths.
- Others
Cobapress is a mixed process of casting and forging , which is specially designed for aluminum and is often used in the automotive industry. Modern rolling mills are very expensive, but also productive.
Machining processes
For machining which is one of turning , drilling and milling . Aluminum materials are easy to machine. However, their exact properties depend on the alloy and structure. It should be noted that the temperatures occurring during processing can quickly reach the melting point. With the same cutting parameters as with steel, however, aluminum results in lower mechanical and thermal stress. When cutting material is often hard metal for eutectic used or diamond for highly abrasive hypereutectic alloys. The machining of anodized workpieces in particular requires hard tools in order to avoid wear and tear from the hard anodized layer. The grinding dust produced when grinding aluminum can lead to an increased risk of explosion.
Welding and soldering
In principle, all aluminum materials are suitable for welding, but pure aluminum tends to have pores in the weld seam. In addition, the aluminum melt tends to react with the atmosphere, which is why welding is almost always carried out under protective gas . MIG and plasma welding as well as TIG welding are well suited . In the latter case, the inert gas argon is used as protective gas when alternating current is used, and helium in the case of direct current .
Both carbon dioxide and solid-state lasers are suitable for laser welding , but not for all alloys. Due to the high thermal conductivity, the melt solidifies very quickly, so that the weld seam tends to develop pores and cracks. The resistance spot welding requires, compared with steel, higher electrical currents and shorter welding times and some special equipment, since the conventional welding equipment for steel are not suitable for it. All alloys are suitable for electron beam welding , but magnesium and tin tend to evaporate during the welding process. Manual arc welding is rarely used, mostly for reworking castings. Soldering is difficult in air because of the oxide layer that forms. Both hard and soft soldering with special fluxes are used . Alternatively, aluminum can be soldered with ultrasound without flux , the oxide layer being broken up mechanically during the soldering process.
Aluminum in nature and organisms
Aluminum in the human body
Aluminum is not an essential trace element and is considered to be dispensable for human nutrition. The human body contains an average of 50 to 150 milligrams of aluminum. About 50 percent of these are distributed to the lung tissue, 25 percent to the soft tissues and another 25 percent to the bones. Aluminum is therefore a natural component of the human body.
99 to 99.9 percent of the amount of aluminum normally ingested in food (10 to 40 mg per day) is excreted unabsorbed in the faeces. Chelating agents (complexing agents) such as citric acid can increase absorption to 2 to 3 percent. The uptake of aluminum salts via the gastrointestinal tract is low; however, it varies depending on the chemical compound and its solubility, the pH value and the presence of complexing agents. It is estimated that 1 ‰ or 3 ‰ of the aluminum contained in food or drinking water is absorbed in the gastrointestinal tract.
From there it gets into numerous tissues and into the blood. Al 3+ in the blood is predominantly (around 80%) bound to transferrin . 16 percent are in the form [Al (PO 4 ) (OH)] - , 1.9 percent as citrate complex , 0.8 percent as Al (OH) 3 , and 0.6 percent as [Al (OH) 4 ] - before . The blood of newborns already contains aluminum ions that come from the maternal circulation. The serum concentrations of about 6-10 μg / l roughly correspond to those of adults. Water-soluble aluminum salts also reach the brain through the blood: the passage at the blood-brain barrier occurs through endocytosis by means of the transferrin receptor and through active, ATP- dependent transport of the citrate. This has been demonstrated in animal experiments using radioactively labeled aluminum of the isotope 26 Al, which does not occur in nature.
The elimination of water-soluble aluminum salts that have entered the organism takes place within a few days mainly through the kidneys through the urine and less through the feces. The half-life in the blood is 8 hours. In dialysis patients with impaired kidney function there is therefore an increased risk of accumulation in the body (brain, bones) with toxic effects such as softening of the bones and damage to the central nervous system ; In addition, dialysis patients are exposed to a higher intake of aluminum due to the pharmaceutical products they need ( phosphate binders ). Aluminum that is not excreted by the kidneys ends up in the bones. There it is eliminated comparatively very slowly (half-life several years), so that model estimates assume that about 1–2% of the absorbed dose accumulates in the body. In a lifetime, around 35 to 50 mg of aluminum accumulate in the body.
plants
Aluminum in the form of various salts (phosphates, silicates) is a component of many plants and fruits, because dissolved aluminum compounds are absorbed by the plants through rain from the soil; this is more the case when the soil is exposed to acid as a result of acid rain (see also forest damage ) .
Much of the world's soil is chemically acidic . If the pH is below 5.0, Al 3+ ions are absorbed by the roots of the plants. This is the case for half of the arable land in the world. In particular, the ions damage the root growth of the fine roots . If the plant is not aluminum tolerant then it is under stress . Numerous enzymes and signaling proteins are affected; the consequences of the poisoning are not yet fully understood. In acidic metal-containing soils, Al 3+ is the ion with the greatest potential for damage. From the model plant Arabidopsis are transgenic known, the mark up their aluminum tolerance and even with crops tolerant varieties are known.
Acid rain, for example, acidified the lakes in Sweden in the 1960s, causing more Al 3+ ions to dissolve and sensitive fish to perish. In Norway, this connection was established during a research project in the 1970s.
At pH values above 5.0, aluminum is bound as a polymeric hydroxyl cation to the surface of silicates . The proportion of mobile cations increases at pH values of 4.2 to 5 .
When the sulfuric acid concentration increases due to acid rain, aluminum hydroxysulphate is formed :
In food
| Food | Content in mg / kg |
|---|---|
| Tea (dry products) | 385 |
| Thyme leaves | 212 |
| Cocoa and chocolate | 100 |
| Types of salad | 28.5 |
| legumes | 22.5 |
| Grain | 13.7 |
| Canned mushrooms | 9.3 |
| Types of cabbage | 9.0 |
| Sausages | 7.8 |
| Canned vegetables | 7.6 |
| Canned fruit | 3.6 |
| Fish and fish products | 3.3 |
| fruit | 3.1 |
| Infant nutrition | 3.0 |
| cheese | 2.9 |
| Fresh mushrooms | 2.7 |
| Peppers, cucumber, tomatoes, melons | 2.2 |
| Potatoes | 2.1 |
| flesh | 1.2 |
Most foods contain trace amounts of aluminum. Unprocessed plant-based foods contain an average of less than 5 mg / kg in the fresh mass. The values vary considerably due to different varieties, growing conditions and origins. For example, lettuce and cocoa have significantly higher average values . Between 5 and 10 mg / kg can be found in bread, cakes, baked goods, a variety of farinaceous foods, some vegetables or sausages. Black tea can have contents of up to 1042 mg / kg in dry matter. However, there the aluminum is bound to poorly absorbable polyphenols , so that absorption in the gastrointestinal tract is made more difficult. Herbs and spices, such as thyme leaves, have a high aluminum content . In a European comparison there are fluctuations, which is probably due to different levels of aluminum base load and the use of aluminum-containing additives.
According to an estimate, cooking or storage in aluminum dishes or in aluminum foil can lead to a maximum additional intake of 3.5 mg / day / person (except for acidic foods). With acidic foods such as sauerkraut or tomatoes, significantly higher values can be achieved due to the acid solubility. The Federal Institute for Risk Assessment (BfR) advises against the preparation and storage of acidic and salty foods in particular in uncoated aluminum containers or aluminum foil. High levels of stress arise, for example, when fish or meat dishes with lemon or other sour ingredients are served in aluminum dishes or foils and are heated to a high temperature for a long time.
With an average of 0.2-0.4 mg / l, drinking and mineral water have low levels in contrast to food and thus only make a small contribution to the daily aluminum intake. The Drinking Water Ordinance sets a limit value of 0.2 mg / l. Drinking water must not have higher values in Germany, Austria and Switzerland.
It is estimated that adult Europeans ingest between 1.6 and 13 mg of aluminum per day on average through their diet. This corresponds to a weekly intake of 0.2 to 1.5 mg aluminum per kg body weight in an adult weighing 60 kg. The great uncertainties are based on the different eating habits and the variable content of aluminum in food, also within a country based on various surveys. If infants are fed with ready-made food, the aluminum concentration in the blood can be 15 μg / l. A possible health damage is not known.
The European Food Safety Authority (Efsa) specifies a tolerable weekly intake (TWI) of 1 mg aluminum per kg body weight, previously it was 7 mg Al per kg KW. Because of the potential for accumulation in the body, Efsa prefers the TWI as opposed to the tolerable daily intake (TDI).
As a food additive under the designation E 173, aluminum is only permitted as a coloring agent for coatings on sugar confectionery and as a decoration for cakes and biscuits. Furthermore, aluminum is approved for coloring drugs and cosmetics. In the investigation of pretzels (pretzels, rods, rolls) from bakeries aluminum has been demonstrated that enters the food when used in the production of pretzels aluminum sheets.
While beer is transported in aluminum barrels, the material aluminum has not established itself for transporting wine . Short-term contact does no harm, but wine defects in smell and taste or as cloudiness can occur after longer contact, especially when standing open in the air.
toxicity
In the case of impaired kidney function and dialysis patients, the intake of aluminum leads to progressive encephalopathy (memory and language disorders, listlessness and aggressiveness) due to the destruction of brain cells and progressive dementia, osteoporosis ( arthritis ) with bone fractures and anemia (because aluminum has the same storage proteins like iron). This was observed in the 1970s in long-term hemodialysis patients who were exposed to a large amount of aluminum (“Dialysis Encephalopathy Syndrome”) .
The health effects of aluminum are discussed controversially, especially with regard to its use in deodorants and food additives. Aluminum has been controversially linked as a factor in connection with Alzheimer's disease .
According to a study by the Federal Institute for Risk Assessment (BfR) from July 2007, no Alzheimer's risk from aluminum from consumer goods was recognized in the general case at the time the study was carried out due to the comparatively small amount; However, as a precautionary measure, acidic foods should not be kept in contact with aluminum pots or foil. The Federal Institute for Risk Assessment (BfR) created a new health risk assessment for aluminum-containing antiperspirants in 2020. The result: According to current scientific knowledge, adverse health effects from the regular use of antiperspirants containing aluminum chlorohydrate are unlikely. When assessing the risk of aluminum, however, it is fundamentally important to consider the total intake via the various entry pathways such as food or products containing aluminum that come into contact with food.
The British Alzheimer's Society, based in London, takes the position that the studies carried out up to 2008 did not convincingly demonstrate a causal relationship between aluminum and Alzheimer's disease. Nevertheless, there are some studies, such as the PAQUID cohort study in France, with a health data analysis of 3777 people aged 65 and over from 1988 to the present, in which aluminum exposure is indicated as a risk factor for Alzheimer's disease. Accordingly, many senile plaques with elevated aluminum levels were found in the brains of Alzheimer's patients. However, it is unclear whether the aluminum accumulation is a consequence of Alzheimer's disease, or whether aluminum is causally related to Alzheimer's disease. The German Alzheimer's Society does not see a convincing connection between aluminum intake and Alzheimer's disease, but admits that aluminum has harmful effects on the brain and states that so far (as of 2013) there has been no clarification of the question of whether aluminum triggers the Alzheimer's disease can be seen. Finally, it is advised that dialysis patients should ensure that only aluminum-free fluids are used to clean the blood and that aluminum-containing drugs that bind gastric acid should only be taken as directed by a doctor.
Aluminum is one of the non-essential trace elements, the toxicity essentially depends on the amount: 10 µg / l aluminum in the blood is considered the normal value, values over 60 µg / l indicate excessive exposure and values over 200 µg / l in the blood are considered toxic. Animal studies using 26 Al show that the serum concentration of aluminum increases by an adjuvanted vaccination by only a few per thousand (from about 5 µg / l to 5.04 µg / l).
Aspects of the life cycle assessment
The production of aluminum is very energy-intensive . Between 12.9 and 17.7 kWh of electrical energy are required for fused-salt electrolysis alone to extract one kilogram of aluminum, depending on the date of construction and the modernity of the plant . When generating electricity for the production of one kilogram of aluminum, 8.4 kg of CO 2 are released in the German power plant park , around 10 kg on average worldwide. However, it should also be considered that due to the cost factor of energy, electrolysis is increasingly taking place in places where cheap, low-CO 2 -emission hydropower can be used, such as in Brazil , Canada , Venezuela or Iceland . However, even with the use of electricity from completely regenerative energies, the production of aluminum is not CO 2 -free, since the oxygen produced during fused- salt electrolysis reacts with the carbon of the electrodes to form CO 2 . The consumption values for raw aluminum increase due to the transport and processing shares for remelting, casting, grinding, drilling and polishing to 16.5 kg CO 2 per kg aluminum consumer good.
The Europe-wide recycling rate for aluminum is 67 percent. In Austria (according to a study from the year 2000) 16,000 tons of aluminum per year are consumed through packaging , and 16,000 tons of aluminum end up in household waste without being recycled (this also includes household aluminum films that are not considered "packaging"). 66 percent of the packaging in the residual waste is aluminum [beverage] cans . These are still metallic in the ashes after incineration and make up an average of 2.3 percent of ash in Europe. In the EU, an average of 70 percent of the aluminum contained in bottom ash is recovered.
The mining of the ore bauxite takes up large areas that can only be used again after recultivation . To produce one ton of aluminum, four tons of bauxite are required. This creates ten tons of overburden. In addition, the production of aluminum oxide using the Bayer process produces around three tons of iron-rich, alkaline red mud , which is hardly recycled and whose landfill or other "disposal" poses major environmental problems (see corresponding sections under red mud and bauxite mining in Australia ).
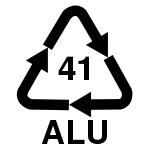
On the other hand, the good reusability of aluminum should be emphasized positively, whereby the residues must be strictly separated and cleaned ( aluminum recycling , recycling code -41 (ALU)). Aluminum is easier to recycle than plastics , but due to downcycling, if it is not sorted according to type , it is somewhat less recyclable than steel . When recycling aluminum, only 5 percent of the amount of energy used in primary production is required. By lightweight aluminum materials (such as aluminum foam, extruded profiles) is the mass of moving parts and vehicles saved, which can lead to the saving of fuel.
Due to its self- passivation, aluminum is more corrosion - resistant than iron and therefore requires fewer corrosion protection measures.
proof
Aluminum salts can be detected by annealing with a dilute cobalt nitrate solution (Co (NO 3 ) 2 ) on the Magnesia channel. This creates the pigment Thénards Blue , a cobalt aluminum spinel with the formula CoAl 2 O 4 . It is also called cobalt blue or cobalt blue, Dumonts blue, celestine blue, cobalt aluminate or - after the discoverer of the pigment, Josef Leithner - Leithner's blue.
Proof by means of a cryolite sample
The sample solution is made alkaline to precipitate aluminum as aluminum hydroxide Al (OH) 3 . The precipitate is filtered off and a few drops of phenolphthalein are added, then washed until there is no longer any red color due to phenolphthalein. If solid sodium fluoride (NaF) is then sprinkled on the precipitate, hydroxide ions , which are released during the formation of cryolite Na 3 [AlF 6 ], cause the phenolphthalein to turn red again.
Evidence as a fluorescent Morin lake
Hydrochloric acid (HCl) is added to the sample and any aluminum present is thus dissolved. Then the sample solution is made strongly alkaline with potassium hydroxide (KOH). If you put a few drops of the sample solution together with the same amount of Morin solution on a spot plate and then acidify with concentrated acetic acid ( glacial acetic acid , CH 3 COOH), green fluorescence can be observed under UV radiation (λ = 366 nm) . The detection is reliable when this fluorescence disappears again when hydrochloric acid is added.
The reason for this is that Al (III) forms a fluorescent colloidal suspension in neutral and acetic acid solutions in conjunction with Morin.
links
- Alumina Al 2 O 3 (English alumina ), also known as alumina or corundum known, is a white powder or in the form of very hard crystals before. It is the end product of the Bayer process and is primarily used as the starting material for aluminum production ( fused- salt electrolysis ). It is also used as a grinding or polishing agent and for watch stones, drawing dies and nozzles. In ceramic form it is used as an insulating material, construction ceramics , as a substrate material for thick-film circuits , as a base plate for power semiconductors and, in transparent form, as a discharge vessel for high-pressure sodium lamps .
- Aluminum hydroxide Al (OH) 3 is also obtained using the Bayer process and is the most important starting material for the production of other Al compounds, especially for aluminates . As a pure product, it is used as a filler and for fire protection in plastics and coatings.
- Aluminum chloride , polyaluminum chloride and aluminum sulfate are mainly used as flocculants in water treatment , wastewater treatment and the paper industry.
- Sodium aluminate NaAl (OH) 4 is also used as a flocculant and is also the raw material for zeolite production, titanium dioxide coating and calcium aluminate sulfate production.
- Zeolites ( aluminosilicates ) as ion exchangers , in foods and in detergents for water softening .
- Alums ( potassium aluminum sulfate , KAl (SO 4 ) 2 · 12H 2 O). Because of its astringent effect it is used as a razor stick to stop small bleeding.
- Aluminum diacetate , known as acetic clay for anti-inflammatory compresses.
- Organoaluminum compounds such as triethylaluminum are used on an industrial scale as catalysts in polyethylene production. Another field of application is semiconductor technology . Here, volatile aluminum alkyls ( trimethylaluminum , triethylaluminum) are used as precursors to the CVD ( chemical vapor deposition ) of aluminum oxide, which is used as an insulator and substitute for the insufficiently insulating silicon dioxide .
- Aluminum oxynitride is a transparent ceramic material.
- Aluminum nitride is a construction and insulation material and is characterized by its very high thermal conductivity at room temperature. In addition, the high band gap could enable the application as a wide band gap semiconductor .
- Lithium aluminum hydride (LiAlH 4 ) is a powerful reducing agent which is widely used in the synthesis of organic compounds .
-
Phosphates : Aluminum phosphates are aluminum salts of phosphoric acid . Due to the nature of the phosphoric acid or the phosphate - anion (PO 4 3- ), under certain conditions water to cleave and as a result polymerize , various aluminum phosphates are known:
- Aluminum orthophosphate (AlPO 4 )
- Aluminum metaphosphate (Al (PO 3 ) 3 )
- Monoaluminium phosphate (Al (H 2 PO 4 ) 3 )
- Aluminum polyphosphate
- In nature, aluminum phosphates usually occur in the form of double salts. Examples include wavellite (Al 3 (PO 4 ) 2 (F, OH) 3 · 5H 2 O) or turquoise , a mixed phosphate made from copper and aluminum / iron: Cu (Al, Fe) 6 (PO 4 ) 4 (OH) 8 · 4 H 2 O. Under special conditions, aluminum appears monovalent. These compounds are used to obtain high-purity aluminum ( subhalide distillation ).
See also
literature
About history
- Hans Joliet (ed.): Aluminum - The first hundred years. VDI Verlag, 1988, ISBN 3-18-400802-9 .
German specialist literature
- Friedrich Ostermann: Application technology aluminum. 3. Edition. Springer, 2014, ISBN 978-3-662-43806-0 .
-
Aluminum paperback . Aluminum-Verlag, Düsseldorf:
- Volume 1: Fundamentals and materials , 16th edition, 2002.
- Volume 2: Forming aluminum materials, casting aluminum parts, surface treatment of aluminum, recycling and ecology , 15th edition 1999, 672 pp.
- Volume 3: Further processing and application , 16th edition, 2003, 863 pp.
- Luitgard Marschall: aluminum. Modern metal. Oekom, Munich 2008, ISBN 978-3-86581-090-8 .
English specialist literature
- George E. Totten, D. Scott MacKenzie: Handbook of Aluminum . Marcel Dekker, Yew York, Basel:
- Volume 1: Physical Metallurgy and Processes . 2003, 1296 pages
- Volume 2: Alloy Production and Materials Manufacturing . 2003, 724 pages
- Joseph R. Davis (Ed.): Aluminum and Aluminum Alloys . 4th edition, 1998, 784 pages
- Calvin C. Willhite et al .: Systematic review of potential health risks posed by pharmaceutical, occupational and consumer exposures to metallic and nanoscale aluminum, aluminum oxides, aluminum hydroxide and its soluble salts . In: Critical reviews in toxicology . tape 44 , Suppl 4, October 2014, p. 1–80 , doi : 10.3109 / 10408444.2014.934439 , PMID 25233067 , PMC 4997813 (free full text).
Web links
- Federal Institute for Risk Assessment (BfR) : Questions and answers on aluminum in food and consumer products
- MATERIAL ARCHIVE: Aluminum - Extensive material information and images.
- News site of the general association of the aluminum industry
- That is really how harmful aluminum is. In: quarks.de . September 27, 2018, accessed on May 31, 2019 (German).
- Bayerischer Rundfunk (Hrsg.): Aluminum: Dangerous for health? January 16, 2019 ( br.de [accessed on May 31, 2019]).
- Lars Fischer: How dangerous is aluminum? Spektrum.de, July 14, 2014.
Individual evidence
- ↑ a b Harry H. Binder: Lexicon of the chemical elements. S. Hirzel Verlag, Stuttgart 1999, ISBN 3-7776-0736-3 .
- ↑ The values for the properties (info box) are taken from www.webelements.com (aluminum) , unless otherwise stated .
- ↑ CIAAW, Standard Atomic Weights Revised 2013 .
- ^ IUPAC, Standard Atomic Weights Revised 2013 (Excel table) .
- ↑ Manjeera Mantina, Adam C. Chamberlin, Rosendo Valero, Christopher J. Cramer, Donald G. Truhlar: Consistent van der Waals Radii for the Whole Main Group. In: Journal of Physical Chemistry A . 113, 2009, pp. 5806-5812, doi: 10.1021 / jp8111556 .
- ↑ a b c d e Entry on aluminum in Kramida, A., Ralchenko, Yu., Reader, J. and NIST ASD Team (2019): NIST Atomic Spectra Database (ver. 5.7.1) . Ed .: NIST , Gaithersburg, MD. doi : 10.18434 / T4W30F ( https://physics.nist.gov/asd ). Retrieved June 11, 2020.
- ↑ a b c d e Entry on aluminum at WebElements, https://www.webelements.com , accessed on June 11, 2020.
- ↑ Aluminum-Taschenbuch - Volume 1 , 16th edition, Aluminum-Verlag, Düsseldorf 2002, p. 74.
- ↑ Robert C. Weast (Ed.): CRC Handbook of Chemistry and Physics . CRC (Chemical Rubber Publishing Company), Boca Raton 1990, ISBN 0-8493-0470-9 , pp. E-129 to E-145. (Values there are based on g / mol and are given in cgs units. The value given here is the SI value calculated from it without measuring units).
- ^ A b A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 91st – 100th, improved and greatly expanded edition. Walter de Gruyter, Berlin 1985, ISBN 3-11-007511-3 , p. 868.
- ↑ a b Yiming Zhang, Julian RG Evans, Shoufeng Yang: Corrected Values for Boiling Points and Enthalpies of Vaporization of Elements in Handbooks. In: Journal of Chemical & Engineering Data . 56, 2011, pp. 328-337, doi: 10.1021 / je1011086 .
- ↑ WB Frank, WE Haupin, H. Vogt, M. Bruno, J. Thonstad, RK Dawless, H. Kvande, OA Taiwo: aluminum. In: Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH, Weinheim 2009, doi : 10.1002 / 14356007.a01_459.pub2 .
- ^ Joseph L. Rose: Ultrasonic Waves in Solid Media . Cambridge University Press, 2004, ISBN 978-0-521-54889-2 , pp. 44 ( limited preview in Google Book search).
- ↑ Tribikram Kundu: Ultrasonic and Electromagnetic NDE for Structure and Material Characterization . CRC Press, 2012, ISBN 978-1-4398-3663-7 , pp. 94 ff . ( limited preview in Google Book search).
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . CRC Press, 1998, ISBN 0-8493-0479-2 .
- ^ Aluminum-Taschenbuch - Volume 1 , 16th edition, Aluminum-Verlag, Düsseldorf 2002, p. 77.
- ↑ T. Merkel, K.-H. Thomas: Taschenbuch der Werkstoffe , 7th improved edition, Carl Hanser Verlag, Munich 2008, p. 294.
- ^ Entry on aluminum, powder, not stabilized in the GESTIS substance database of the IFA , accessed on August 9, 2016(JavaScript required) .
- ^ Entry on aluminum, powder, phlegmatized in the GESTIS substance database of the IFA , accessed on August 9, 2016(JavaScript required) .
- ↑ Entry on aluminum in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Data sheet aluminum at AlfaAesar, accessed on March 13, 2011 ( PDF )(JavaScript required) .
- ↑ a b George E. Totten, D. Scott MacKenzie: Handbook of Aluminum Volume 1: Physical Metallurgy and Processes. Marcel Dekker, Yew York, Basel. 2003, pp. 33-34.
- ^ Alumina Production. In: world-aluminium.org. The International Aluminum Institute, accessed February 14, 2016 .
- ↑ Primary Aluminum Production. In: world-aluminium.org. The International Aluminum Institute, accessed February 14, 2016 .
- ^ Norman N. Greenwood, Alan Earnshaw: Chemistry of the elements. Wiley-VCH, Weinheim 1988, ISBN 3-527-26169-9 .
- ↑ NA Figurowski: The discovery of the chemical elements and the origin of its name , in German translation of Leo Korniljew / Ernst Lemke, Moscow 1981, ISBN 3-7614-0561-8 , S. 64th
- ^ Norman N. Greenwood, Alan Earnshaw: Chemistry of the Elements. 2nd Edition. Butterworth – Heinemann, 1997, ISBN 0-08-037941-9 , p. 217.
- ↑ Mineral Species containing Aluminum (Al) on Webmineral.
- ↑ BV Oleinikov, AV Okrugin, NV Leskova: Petrological significance of the occurrence of native aluminum in basic rocks . In: Doklady Akademii Nauk SSSR . tape 243 , 1978, pp. 191–194 ( rruff.info [PDF; 240 kB ; accessed on January 11, 2018]).
- ↑ Michael Fleischer , Adolf Pabst , JA Mandarino : New mineral names . In: American Mineralogist . tape 65 , 1980, pp. 205–210 ( rruff.info [PDF; 1.1 MB ; accessed on January 11, 2018]).
- ↑ Find location list for aluminum in the Mineralienatlas and Mindat
- ^ IMA / CNMNC List of Mineral Names; November 2018 (PDF 1.7 MB; aluminum see p. 7)
- ^ IMA / CNMNC List of Mineral Names; 2009 (PDF 1.8 MB, aluminum see p. 7).
- ↑ a b Stefan Weiß: The large Lapis mineral directory. All minerals from A - Z and their properties. Status 03/2018 . 7th, completely revised and supplemented edition. Weise, Munich 2018, ISBN 978-3-921656-83-9 .
- ^ David Barthelmy: Minerals Arranged by the New Dana Classification. 01/01/01 Gold group. In: webmineral.com. Retrieved January 14, 2019 .
- ↑ a b aluminum . In: John W. Anthony, Richard A. Bideaux, Kenneth W. Bladh, Monte C. Nichols (Eds.): Handbook of Mineralogy, Mineralogical Society of America . 2001 ( handbookofmineralogy.org [PDF; 57 kB ; accessed on January 11, 2018]).
- ^ Rudolf Debar: The aluminum industry. 2nd Edition. Springer 1925, pp. 55 ff. ( Limited preview in the Google book search).
- ^ Eutectic aluminum oxide / cryolite. ( Memento from April 19, 2015 in the Internet Archive )
- ↑ Aluminum and silicon: from storage to use. ( Memento from January 30, 2012 in the Internet Archive ) (PDF, seminar paper; 527 kB) p. 10.
- ↑ a b Matthias Dienhart: Holistic balancing of the energy supply for aluminum production. (PDF; 1.3 MB) Dissertation . Rheinisch-Westfälische Technische Hochschule Aachen , June 2003, p. 7.
- ↑ Rainer Quinkertz: Optimizing the use of energy in aluminum production. Dissertation. Rheinisch-Westfälische Technische Hochschule Aachen , 2002, pp. 75–77.
- ↑ United States Geological Survey: World Alumina Refinery and Bauxite Mine Production and Bauxite Reserves
- ↑ United States Geological Survey: World Smelter Production and Capacity.
- ↑ R. Feige, G. Merker: SEROX - a synthetic Al glass raw material. (PDF).
- ↑ Recycling is only the second best way . In: Der Spiegel . No. 25 , 1993 ( online ).
- ↑ Udo Boin, Thomas Linsmeyer, Franz Neubacher, Brigitte Winter: State of the art in secondary aluminum production with regard to the IPPC directive . (Austrian) Federal Environment Agency, Vienna 2000, ISBN 3-85457-534-3 . ( Umweltbundesamt.at PDF)
- ^ Ralph WG Wyckoff: Crystal Structures . 2nd Edition. tape 1 . John Wiley & Sons, New York, London, Sydney 1963, pp. 3 (in the appendix ).
- ^ Hugo Strunz , Ernest H. Nickel : Strunz Mineralogical Tables. Chemical-structural Mineral Classification System . 9th edition. E. Schweizerbart'sche Verlagbuchhandlung (Nägele and Obermiller), Stuttgart 2001, ISBN 3-510-65188-X , p. 35 .
- ^ Friedrich Ostermann: Application technology aluminum . 3. Edition. Springer, 2014, pp. 71–78.
- ↑ a b c d e f g h i j k l m Friedrich Ostermann: Application technology aluminum . 3. Edition. Springer, 2014, p. 212.
- ^ Friedrich Ostermann: Application technology aluminum . 3. Edition. Springer, 2014, pp. 212-213.
- ↑ a b D. R. Askeland: Materials Science. Spectrum, Heidelberg 1996, ISBN 3-86025-357-3 .
- ^ Aluminum-Taschenbuch - Volume 1 , 16th edition, Aluminum-Verlag, Düsseldorf 2002, p. 79.
- ^ Aluminum-Taschenbuch - Volume 1 , 16th edition, Aluminum-Verlag, Düsseldorf 2002, p. 77.
- ↑ Wolfgang Weißbach: Materials science, structures, properties, testing . Springer-Verlag, 2012, ISBN 978-3-8348-8318-6 , p. 196 ( limited preview in Google Book search).
- ↑ Bernhard Ilschner: Material Science and Manufacturing Technology Properties, Processes, Technologies . Springer, Berlin 2010, ISBN 978-3-642-01734-6 , pp. 277 .
- ^ Friedrich Ostermann: Application technology aluminum . 3. Edition. Springer, 2014, p. 214.
- ^ Friedrich Ostermann: Application technology aluminum . 3. Edition. Springer, 2014, pp. 212-214.
- ^ Friedrich Ostermann: Application technology aluminum . 3. Edition. Springer, 2014, pp. 212, 214.
- ↑ The ELB technology guide. In: Eloxalwerk Ludwigsburg.
- ↑ What are dangerous goods in passenger baggage? ( Memento from August 18, 2017 in the Internet Archive ) Website of the Federal Aviation Office.
- ^ Aluminum-Taschenbuch - Volume 1 , 16th edition, Aluminum-Verlag, Düsseldorf 2002, pp. 98–99, 167–168.
- ^ Aluminum-Taschenbuch - Volume 1 , 16th edition, Aluminum-Verlag, Düsseldorf 2002, pp. 189–190.
- ^ Aluminum-Taschenbuch - Volume 1 , 16th edition, Aluminum-Verlag, Düsseldorf 2002, pp. 167–168.
- ^ Aluminum-Taschenbuch - Volume 1 , 16th edition, Aluminum-Verlag, Düsseldorf 2002, p. 99.
- ↑ Aluminum-Taschenbuch - Volume 1 , 16th edition, Aluminum-Verlag, Düsseldorf 2002, pp. 117-118.
- ^ Aluminum-Taschenbuch - Volume 1 , 16th edition, Aluminum-Verlag, Düsseldorf 2002, pp. 102-103.
- ^ Aluminum-Taschenbuch - Volume 1 , 16th edition, Aluminum-Verlag, Düsseldorf 2002, pp. 152–153.
- ↑ Aluminum-Taschenbuch - Volume 1 , 16th edition, Aluminum-Verlag, Düsseldorf 2002, pp. 125–126.
- ↑ Aluminum-Taschenbuch - Volume 1 , 16th edition, Aluminum-Verlag, Düsseldorf 2002, pp. 142-143.
- ↑ Aluminum-Taschenbuch - Volume 1 , 16th edition, Aluminum-Verlag, Düsseldorf 2002, p. 153.
- ↑ Statistics. Wirtschaftsvereinigung Stahl, accessed on July 24, 2017 .
- ^ Price index on the London Metal Exchange
- ^ DR Askeland: Material science. Spektrum, Heidelberg 1996, p. 364.
- ^ DR Askeland: Material science. Spektrum, Heidelberg 1996, p. 362.
- ↑ Gesamtverband der Aluminiumindustrie e. V .: Seawater resistance of wrought aluminum alloys. (PDF; 54 kB) (No longer available online.) Archived from the original on May 13, 2016 ; Retrieved March 29, 2013 .
- ↑ world-aluminium.org: The Global Aluminum Industry 40 years from 1972 (PDF; 308 kB), accessed on November 17, 2013.
- ↑ Sales markets. General Association of the Aluminum Industry, accessed on July 24, 2017 .
- ↑ a b Friedrich Ostermann: Application technology aluminum . 3. Edition. Springer, 2014, pp. 34–39.
- ^ Friedrich Ostermann: Application technology aluminum . 3. Edition. Springer, 2014, pp. 39–41.
- ^ Friedrich Ostermann: Application technology aluminum . 3. Edition. Springer, 2014, pp. 52–57.
- ↑ René Flosdorff , Günther Hilgarth: Electrical Distribution . 8th edition. Teubner, 2003, ISBN 3-519-26424-2 , chapter 1.2.2.4.
- ^ Stanley Wolf: Silicon Processing for the VLSI Era. Volume 2: Process Integration . Lattice Press, Sunset Beach, Calif. 1990, ISBN 0-9616721-4-5 , pp. 191 ff .
- ^ Stanley Wolf: Silicon Processing for the VLSI Era. Volume 4: Deep-Submicron Process Technology . Lattice Press, Sunset Beach 2002, ISBN 0-9616721-7-X , pp. 723 ff .
- ↑ cf. Stanley Wolf: Silicon Processing for the VLSI Era. Volume 4: Deep-Submicron Process Technology . Lattice Press, Sunset Beach 2002, ISBN 0-9616721-7-X , pp. 713 .
- ^ Friedrich Ostermann: Application technology aluminum . 3. Edition. Springer, 2014, pp. 63–64.
- ↑ Robert Brockmann: Loss of the helium tightness . In: Vacuum in research and practice . tape 26 , no. 2 , April 1, 2014, p. 19–22 , doi : 10.1002 / vipr.201400547 ( PDF ).
- ^ Healing of aluminum: Current information on the UST process.
- ^ Friedrich Ostermann: Application technology aluminum . 3. Edition. Springer, 2014, p. 65.
- ↑ How environmentally friendly is the european aluminum industry
- ↑ Joachim Achtziger , Günter Pfeifer , Rolf Ramcke , Konrad Zilch : Masonry Atlas . Institute for International Architecture Documentation, 2001, p. 59 ( limited preview in Google Book search).
- ↑ K. Zilch, CJ Diederichs, R. Katzenbach, KJ Beckmann (eds.): Handbook for civil engineers. Technology, organization and economy . 2nd Edition. Springer, 2012, ISBN 978-3-642-14449-3 , pp. 182 .
- ^ Friedrich Ostermann: Application technology aluminum . 3. Edition. Springer, 2014, pp. 57–59.
- ↑ Alcoa Architectural Products (Ed.): Aluminum composite panels and sheets: Forming, designing, inspiring . September 30, 2013 ( arconic.com [PDF; 720 kB ; accessed on June 21, 2017] leaflet).
- ↑ Wolfgang Hoferer: Slugs made of aluminum . Ed .: Aluminum-Werke Wutöschingen (= EC safety data sheet in accordance with 91/155 / EEC . EC directive .9.99). May 5, 2008, sheet 4 ( aww.de [PDF; 33 kB ; accessed on June 21, 2017] First version March 29, 2007).
- ↑ Bernd Leitenberger: Chemical rocket fuels, part 1. In: Bernd Leitenberger's Web Site.
- ^ Aluminum on Fireworks Wiki: www.feuerwerk.net .
- ↑ Eberhard Roos , Karl Maile : Material science for engineers , 4th edited edition, 2011, Springer, p. 252.
- ^ A. Herbert Fritz, Günter Schulze: Manufacturing technology. 10th edition. Springer, Berlin 2012, ISBN 978-3-642-29785-4 , p. 36.
- ^ Rüdiger Bähr: Urformen in: Molitor, Grote, Herold, Karpuschewski: Introduction to Manufacturing , Shaker, 2008, p. 19.
- ↑ Heiko Lickfett: Economic importance of the foundry industry in: Andreas Bühring-Polaczek , Walter Michaeli , Günter Spur (eds.): Handbuch Urformen , Hanser, 2014, pp. 13-16.
- ^ Bernhard Ilschner, Robert F. Singer: Materials science and manufacturing technology: properties, processes, technologies. 5th edition. Springer, Berlin 2010, pp. 449-450.
- ^ Friedrich Ostermann: Application technology aluminum . 3. Edition. Springer, 2014, pp. 419-422.
- ↑ Wolfgang Lehnert: Forming of aluminum material . In: Günter Drossel, Susanne Friedrich, Catrin Kammer, Wolfgang Lehnert (eds.): Aluminum Taschenbuch: Volume 2: Forming of aluminum materials, casting of aluminum parts, surface treatment of aluminum, recycling and ecology . 16th edition. Beuth, Berlin 2009, ISBN 978-3-410-22029-9 , pp. 1 ( extract (PDF) [accessed on September 14, 2014]).
- ^ Fritz Klocke, Wilfried König: Manufacturing process 4: Forming. 5th edition. Springer, Heidelberg, pp. 89-90.
- ^ Friedrich Ostermann: Application technology aluminum . 3. Edition. Springer, 2014, pp. 441-450.
- ^ Friedrich Ostermann: Application technology aluminum . 3. Edition. Springer, 2014, pp. 451–462.
- ^ Eberhard Roos, Karl Maile: Materials science for engineers: Fundamentals, application, testing. 4th edition. Springer, Berlin 2011, p. 240.
- ↑ Wilfried König, Fritz Klocke: Manufacturing process 1: turning, drilling, milling. 8th edition. Springer 2008, ISBN 978-3-540-23458-6 , p. 319.
- ↑ Thorsten Zurell: Extraction of aluminum grinding dust and welding fumes in the automotive industry with explosion-proof filter systems. In: Hazardous substances - cleanliness. Air . 62, No. 11/12, 2002, pp. 455-460.
- ↑ Hans J. Fahrenwaldt: Practical knowledge of welding technology: materials, processes, production . 3. Edition. Vieweg + Teubner, Wiesbaden 2008, ISBN 978-3-8348-0382-5 , pp. 205 .
- ↑ Hans J. Fahrenwaldt: Practical knowledge of welding technology: materials, processes, production . 3. Edition. Vieweg + Teubner, Wiesbaden 2008, ISBN 978-3-8348-0382-5 , pp. 71, 206 .
- ↑ Udo M. Spornitz: Anatomy and Physiology. Textbook and atlas for nursing and healthcare professions. Springer, Berlin 2010, ISBN 978-3-642-12643-7 .
- ↑ a b entry on aluminum. In: Römpp Online . Georg Thieme Verlag, accessed on June 12, 2013.
- ↑ a b c d ALUMINUM IN VACCINE . ( arznei-telegramm.de [accessed on October 6, 2018]).
- ↑ a b c Waldemar Ternes: Biochemistry of the elements: Inorganic chemistry of biological processes . Springer DE, 2013, ISBN 978-3-8274-3019-9 ( limited preview in Google book search).
- ↑ a b c d e Doris Oberle et al .: Vaccination Complications and Dealing with Suspected Cases . In: Federal Health Gazette - Health Research - Health Protection . tape 62 , no. 4 , April 1, 2019, p. 450-461 , doi : 10.1007 / s00103-019-02913-1 .
- ↑ a b c d e J. -P. Goullé and L. Grangeot-Keros: Aluminum and vaccines: Current state of knowledge . In: Médecine et Maladies Infectieuses . October 11, 2019, doi : 10.1016 / j.medmal.2019.09.012 .
- ↑ Wilfried Puwein: The "forest dying" in Austria and its economic consequences. No. 11, 1989 ( wifo.ac.at PDF; 792 kB).
- ↑ Hideaki Matsumoto: Cell biology of aluminum toxicity and tolerance in higher plants . In: International Review of Cytology . tape 200 , 2000, pp. 1-46 , doi : 10.1016 / S0074-7696 (00) 00001-2 .
- ↑ Bunichi Ezaki et al .: Different mechanisms of four aluminum (Al) -resistant transgenes for Al toxicity in Arabidopsis . In: Plant Physiology . tape 127 , no. 3 , 2001, p. 918-927 , PMID 11706174 .
- ↑ Charlotte Poschenrieder et al .: A glance into aluminum toxicity and resistance in plants . In: Science of the Total Environment . tape 400 , no. 1–3 , 2008, pp. 356–368 , doi : 10.1016 / j.scitotenv.2008.06.003 .
- ↑ Sanjib Kumar Panda, Frantisek Baluska, Hideaki Matsumoto: Aluminum stress signaling in plants . In: Plant Signaling & Behavior . tape 4 , no. 7 , 2009, p. 592-597 , PMID 19820334 , PMC 2710549 (free full text).
- ↑ Norway's freshwater fish die from acid rain . In: Arbeiter-Zeitung . Vienna September 29, 1981, p. 10 , bottom left ( berufer-zeitung.at - the open online archive - digitized).
- ^ Federal Association of Food Chemists in the Public Service (BLC): Aluminum in food.
- ↑ a b c d e f Sabine Greßler and René Fries: Study: "Aluminum toxicology and health aspects of body-related applications". Federal Ministry of Labor, Social Affairs, Health and Consumer Protection , accessed on August 7, 2019 .
- ↑ a b c d Safety of aluminum from dietary intake - Scientific Opinion of the Panel on Food Additives, Flavors, Processing Aids and Food Contact Materials (AFC) In: The EFSA Journal . 754, 2008, pp. 1-34; doi: 10.2903 / j.efsa.2008.754 .
- ^ Aluminum in food: food.org .
- ↑ a b c Federal Institute for Risk Assessment (Ed.): No Alzheimer's risk from aluminum from consumer goods. (PDF; 106 kB) July 22, 2007.
- ↑ Reducing aluminum intake can minimize potential health risks. In: Opinion No. 045/2019 of the Federal Institute for Risk Assessment (BfR). November 18, 2019, accessed December 1, 2019 . doi : 10.17590 / 20191115-135258 .
- ↑ "Aluminum foil does not belong in the kitchen". Federal institute warns of risks from aluminum in the body. In: tagesspiegel.de. November 19, 2019, accessed December 1, 2019 .
- ↑ Entry on E 173: Aluminum in the European database on food additives, accessed on June 16, 2020.
- ^ Aluminum in the food additive database.
- ↑ Chemisches und Veterinäruntersuchungsamt Karlsruhe: Laugengebäck: How does aluminum get into the baked goods? (PDF) 2004.
- ↑ H. Eschnauer: The use of aluminum in the wine industry. Vitis, 1, 1958, pp. 313-320, cited by p. 319, vitis-vea.de (PDF; 729 kB).
- ↑ HE Müller, W. thin leather, W. Muhlenberg, R. Ruckdeschel: Legionella - an active problem of sanitary hygiene. 3. Edition. expert-Verlag, ISBN 978-3-8169-2725-9 , p. 14 ( limited preview in the Google book search).
- ^ HJ Gitelman: Physiology of Aluminum in Man. In: Aluminum and Health. CRC Press, 1988, ISBN 0-8247-8026-4 , p. 90 ( limited preview in Google book search).
- ^ A b P.C. Ferreira, A. Piai, AM Takayanagui, SI Segura-Muñoz: Aluminum as a risk factor for Alzheimer's disease . In: Revista Latino-Americana de Enfermagem . tape 16 , no. 1 , 2008, p. 151-157 , doi : 10.1590 / S0104-11692008000100023 , PMID 18392545 .
- ↑ Hinnerk Feldwisch-Drentrup and Jakob Simmank: Aluminum salts: The aluminum deodorant hysteria . In: The time . Hamburg December 12, 2019 ( zeit.de [accessed January 28, 2020]).
- ↑ Jakob Kneser : Mr. Aluminum. Gaps in aluminum research. WDR , October 17, 2017, accessed December 21, 2017 .
- ↑ Federal Institute for Risk Assessment (Ed.): New studies on aluminum-containing antiperspirants: Health impairments from aluminum absorption through the skin are unlikely (PDF), July 20, 2020.
- ↑ BfR: Reducing aluminum intake can minimize potential health risks. In: BfR. BfR, accessed on May 8, 2020 .
- ^ Aluminum and Alzheimer's disease. ( Memento of March 11, 2012 in the Internet Archive ) The Alzheimer's Society. Retrieved January 30, 2009.
- ↑ V. Rondeau, H. Jacqmin-Gadda, D. Commenges, C. Helmer, J.-F. Dartigues: Aluminum and Silica in Drinking Water and the Risk of Alzheimer's Disease or Cognitive Decline: Findings From 15-Year Follow-up of the PAQUID Cohort . In: American Journal of Epidemiology . tape 169 , no. 4 , 2008, p. 489-496 , doi : 10.1093 / aje / kwn348 , PMID 19064650 , PMC 2809081 (free full text).
- ↑ Sakae Yumoto, Shigeo Kakimi, Akihiro Ohsaki, Akira Ishikawa: Demonstration of aluminum in amyloid fibers in the cores of senile plaques in the brains of patients with Alzheimer's disease . In: Journal of Inorganic Biochemistry . tape 103 , no. 11 , 2009, p. 1579-1584 , doi : 10.1016 / j.jinorgbio.2009.07.023 , PMID 19744735 .
- ↑ Walter Stehling, Timo Grimmer, Alexander Kurz: Light metal with serious consequences? Aluminum and Alzheimer's Disease. In: Archive Alzheimer Info. DAlzG, March 2013, accessed on October 11, 2019 .
- ↑ H. Lüllmann, K. Mohr, M. Wehling, L. Hein: Pharmakologie und Toxikologie , Thieme Verlag, 2016, ISBN 978-3-13-368518-4 , pp. 622–623.
- ^ Wolfgang Maurer: Vaccine skeptics - vaccine opponents. From another reality on the internet . In: Pharmacy in our time . tape 37 , no. 1 , January 2008, p. 64-70 , doi : 10.1002 / pauz.200700252 .
- ^ Aluminum industry ( Memento from May 2, 2010 in the Internet Archive ) on staufenbiel.de
- ^ Steel in the circular economy - A life cycle perspective. ( Memento of April 12, 2016 in the Internet Archive ) (PDF) p. 16.
- ↑ Hans Daxbeck, Adolf Merl, Eva Ritter, Paul H. Brunner: Analysis of the rivers of the licensed aluminum in Austria . Vienna University of Technology, Institute for Water Quality and Waste Management, 2000 ( rma.at PDF).
- ↑ International Aluminum Journal. No. 6, 2013, p. 81 ff.
- ↑ International Aluminum Journal. No. 91, 2015, p. 53.
- ↑ Uwe Kerkow, Jens Martens, Axel Müller: From ore to the car. ( Memento of October 10, 2015 in the Internet Archive ) (PDF) Aachen / Bonn / Stuttgart 2012, ISBN 978-3-943126-07-5 , p. 29.
- ↑ Manfred Sietz, Stefan Seuring : Life cycle assessment in operational practice. Eberhard Blottner, Taunusstein 1997, p. 103 ( limited preview in the Google book search).
- ^ Case history: The truth about recycling. In: The Economist. 2007.
- ^ J. Strähle, E. Schweda: Jander Blasius - Textbook of analytical and preparative inorganic chemistry , 16th edition, Hirzel, Stuttgart 2006, ISBN 3-7776-1388-6 , p. 626.










![{\ mathrm {Al_ {2} O_ {3} (s) +2 \ Cl ^ {-} (aq) +3 \ H_ {2} O (l) \ longrightarrow 2 \ [Al (OH) _ {2} Cl] (aq) +2 \ OH ^ {-} (aq)}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/a732a2263125aca5df8ea80c46a7974bb87472cc)
![{\ mathrm {Al (s) +4 \ H_ {2} O (l) + Cl ^ {-} (aq) \ longrightarrow [Al (OH) _ {2} Cl] (aq) +3 \ e ^ { -} + 2 \ H_ {3} O ^ {+} (aq)}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/6524b2768718ef36682d719c25015626f764b083)