Pyrite
| Pyrite | |
|---|---|
| Cubic pyrite, Navajún , La Rioja , Spain | |
| General and classification | |
| other names |
|
| chemical formula | FeS 2 |
|
Mineral class (and possibly department) |
Sulfides and sulfosalts |
|
System no. to Strunz and to Dana |
2.EB.05a ( 8th edition : II / C.05) 02.12.01.01 |
| Crystallographic Data | |
| Crystal system | cubic |
| Crystal class ; symbol | cubic-disdodecahedral; 2 / m 3 |
| Space group | Pa 3 (No. 205) |
| Lattice parameters | a = 5.42 Å |
| Formula units | Z = 4 |
| Twinning | Penetration twins, twin axis [001] |
| Physical Properties | |
| Mohs hardness | 6 to 6.5 |
| Density (g / cm 3 ) | 4.95 to 5.2 |
| Cleavage | indistinct after {001} |
| Break ; Tenacity | shell-like, brittle |
| colour | copper to golden yellow |
| Line color | green to bluish black |
| transparency | opaque |
| shine | Metallic luster |
| magnetism | magnetic by heating |
| Other properties | |
| Chemical behavior | soluble in various acids; The substance decomposes on heating above 743 ° C |
| Special features | Striations on cube surfaces |
Pyrite , also known as pebbles , iron pebbles , fool's gold or fool's gold , is a very common mineral from the class of " sulfides and sulfosalts ". From a chemical point of view, it is the cubic modification of iron (II) disulfide with the chemical composition FeS 2 , i.e. it consists of iron and sulfur in a molar ratio of 1: 2.
Pyrite is opaque in every form and develops predominantly idiomorphic crystals in the form of cubes or pentagon dodecahedra . Also octahedron and Disdodekaeder are common as well as combinations between these forms. The crystal surfaces often show characteristic striations and a lively metallic sheen when fresh .
With a Mohs hardness of 6 to 6.5, pyrite is one of the hard minerals that, like the reference mineral orthoclase (6), can just be scratched with a file .
Etymology and history
The name pyrite comes from ancient Greek : from πῦρ pyr for " fire ", πυρίτης (λἱθος) pyrítes (líthos) for "flint" is derived. The background to this is that pyrite splinters can be knocked off with a hard flint , which self-ignite and burn in the air:
This property was already used in the Stone Age to start fires .
For example, pebbles with processing traces were discovered at various sites, which could be assigned to different epochs of the Stone Age. Finds from the Vogelherd cave near Niederstotzingen (Baden-Württemberg) in Germany, a grotto near Les Eyzies-de-Tayac-Sireuil and the Grand Abri near Laussel in the French Dordogne department and the Grotte du Bois Laiterie in the municipality of Profondeville and Trou de Chaleux near Hulsonniaux (municipality of Houyet ) in Belgium from the Upper Paleolithic ; Finds from Trou al'Wesse in the Belgian municipality of Modave from the Mesolithic and finds from Robenhausen , Feldmeilen- Frontfeld, Wollishofen -Haumesser (Canton of Zurich) and Muntelier (Canton of Friborg) in Switzerland, from Lac de Chalain (Jura department), nearby Gisement des Baigneurs in the municipality of Charavines ( Département Isère ) and near Beg-er-Goalennec on the Quiberon peninsula ( Département Morbihan ) in France from the Neolithic .
In ancient times , the term pyrítes included not only the mineral known today as pyrite, but also other “fire-concealing” stones. As described Pliny , for example, in his 36th volume of the Naturalis Historia to texture of the stones two types of metallic aussehendem pyrite of silver or golden color from the mines of Cyprus . It is assumed that the gold-colored one is today's pyrite (possibly including copper pebbles ) and the silver-colored one is another, as yet unidentified sulfide.
The colloquial name Katzengold is derived from the Old High German Kazzūngold , which means "golden-yellow cherry resin ". This designation appeared in manuscripts from the 12th century with the idea of something fake or fake (as opposed to real gold). In the English-speaking world it is called fool's gold , which means " fool's gold ". Pyrite is also called fool's gold in German , but far less often. The term may have been adopted from English. Completely different from this is the rusty gold made of brass .
For a long time pyrite and marcasite were thought to be the same mineral; Both were often referred to in the literature as pebbles or iron pebbles , also called marcasite, and marcasite was a common name until the 18th and 19th centuries. It was not until Wilhelm von Haidinger made it clear in 1845 that iron pebbles actually consisted of two different, albeit very similar minerals and called the hexahedral (cubic) pyrite and the “prismatic” (rhombic) marcasite.
Since pyrite was known and recognized as an independent mineral type long before the founding of the International Mineralogical Association (IMA), this was adopted by its Commission on New Minerals, Nomenclature and Classification (CNMNC) and referred to pyrite as a so-called grandfathered mineral .
classification
Already in the outdated 8th edition of the mineral classification according to Strunz , pyrite belonged to the mineral class of "sulfides and sulfosalts" and there to the department of "sulfides with [the substance ratio] M: S <1: 1", where he named "pyrite Series "with the system no. II / C.05 and the other members Aurostibit , Cattierit , Geversit , Hauerit , Laurit , Michenerit , Penroseit , Sperrylite , Trogtalit , Vaesit and Villamanínit .
In the Lapis mineral directory according to Stefan Weiß, which, out of consideration for private collectors and institutional collections, is still based on this old form of Karl Hugo Strunz's system , the mineral was given the system and mineral number. II / D.17-30 . In the "Lapis system" this also corresponds to the section "Sulphides with [the molar ratio] metal: S, Se, Te <1: 1". Here pyrite forms eponymous the "pyrite group" with the other members Aurostibit, cattierite, Changchengit , Dzharkenit , Erlichmanit , Fukuchilit , Geversit, Hauerit, Insizwait , Krutaite , Laurit, Maslovit , Mayingit , Michenerit, Padmait , Penroseite, Sperrylith, Testibiopalladit , Trogtalit, Vaesit and Villamanínit (as of 2018).
The 9th edition of Strunz's mineral systematics , valid since 2001 and updated by the International Mineralogical Association (IMA) until 2009, initially assigns pyrite to the more general section of "Metal sulfides with [the substance ratio] M: S ≤ 1: 2". However, this is further subdivided according to the exact molar ratio and the predominant metal ions in the compound, so that the mineral can be found according to its composition in the sub-section "M: S = 1: 2, with Fe, Co, Ni, PGE etc." , where the namesake of the still existing "pyrite group" with the system no. 2.EB.05a and the other members Aurostibit, cattierite, Dzharkenit, Erlichmanit, Fukuchilit, Gaotaiit , Geversit, Hauerit, Insizwait, Iridisit (currently not recognized by the IMA), Krutaite, Laurit, Penroseite, Sperrylith, Trogtalit, Vaesit and Villamanínit is.
The systematics of minerals according to Dana , which is mainly used in the English-speaking world , assigns pyrite to the class of "sulphides and sulphosalts" and there in the category of "sulphide minerals". Here it also forms the eponymous "pyrite group (isometric: Pa 3 )" with the system no. 02:12:01 and the other members Aurostibit, cattierite, Dzharkenit, Erlichmanit, Fukuchilit, Gaotaiit, Geversit, Hauerit, Insizwait, Krutaite, Krutovit, Laurit, Mayingit, Penroseite, Sperrylith, Trogtalit, Vaesit and Villamanínit within the subdivision " sulphides - including selenides and tellurides - with the composition A m B n X p , with (m + n): p = 1: 2 “.
Chemism
Theoretically, pyrite consists of iron (Fe) and sulfur (S) in a ratio of 1: 2, i.e. with an ideal composition ( purity of matter ), which corresponds to a mass fraction (% by weight) of 46.55% Fe and 53.45% S. . Natural pyrites with an almost pure composition of 46.49% Fe and 53.49% S and only traces of 0.04% SiO 2 were found on the Italian island of Elba , among other places .
In many mineral samples, however, there are sometimes considerable proportions of other elements. For example, a nickel content of 16.69% was measured in samples from the Millclose mine near South Darley in the English county of Derbyshire and a cobalt content of 13.90% in samples from the copper and cobalt region of Gladhammar near Västervik in the Swedish province of Kalmar County. .
Furthermore, foreign admixtures of arsenic , antimony and thallium and, more rarely, mechanical admixtures such as copper , gold , silver and zinc were found in mineral samples from other sites . Of the elements mentioned, however, only proportions of nickel, cobalt and arsenic can serve as a possible replacement for some of the iron and sulfur, while the rest are based on mechanical admixtures of chalcopyrite ( copper pyrites , CuFeS 2 ).
Due to the high proportion of homeopolar, covalent bonds with a correspondingly high bond energy, pyrite generally shows a rather low tendency to form lattice defects and mixed crystals . Pyrite can only be mixed with cattierite (CoS 2 ) without restriction. Mixed crystals with hauerite (MnS 2 ) are practically non-existent. The solubility of vaesite (NiS 2 ) in pyrite is rather low. According to the analyzes carried out by LA Clark and G. Kullerud in 1963, the maximum solubility of NiS 2 in FeS 2 is 7.7% at 729 ° C. and drops to 6.8% at 700 ° C., but could no longer be determined at lower temperatures will. Most pyrites containing nickel, however, are deposited at relatively low temperatures, the equilibrium solubility of nickel being well below 1% (all data in% by weight). The nickel-containing pyrite variety Bravoite can only form below 137 ° C and most of the nickel in these pyrites is in a metastable solid solution.
Crystal structure
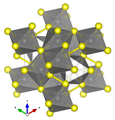
Pyrite crystallizes in the cubic-disdodecahedral crystal class in the space group Pa 3 (space group no. 205) with the lattice parameter a = 5.42 Å (542 pm ) and four formula units per unit cell .
Structurally , the pyrite is very similar to the halite (also sodium chloride , NaCl), although the singly positively charged sodium ions by iron ions and the singly negatively charged chloride ions by dumbbell-shaped S 2 groups, a disulfide ion that structurally corresponds to the peroxide ion, are replaced. The dumbbell axes are each aligned parallel to the 3-fold axes of rotation, but in different orientations, which leads to a reduction in symmetry. Inside the “sulfur dumbbell” there is a covalent bond, whereas between sulfur and iron there is an ionic bond .
| Crystal structure of pyrite |
|
|
| Color table: __ Fe __ S |
Occasionally, the lower symmetry of the disdodecahedral compared to the highly symmetrical hexakisoctahedral class can be recognized by the surface strips of cubic pyrite crystals. The stripes only point in the same direction on the opposite cube faces. The axes of rotation perpendicular to the surfaces of the cube are not four-digit as in the case of a geometrically exact cube , but only two-digit.
The crystal form of the pentagon dodecahedron, which often occurs in pyrite, is a characteristic form of the disdodecahedral class.
characteristics
morphology
Pyrite usually occurs in coarse, granular masses or forms spherical to raspberry-shaped, concentric-shell aggregates. Disk-shaped, radial-ray aggregates (pyrite suns) are formed in sedimentary form.
Well-formed crystals are common and can grow to over 10 inches. So far, more than 60 crystal forms are known. The most common forms with their Miller indices are the cube {100}, the pentagonal dodecahedron {210} and the octahedron {111} and their combinations. The pentagonal dodecahedron is mainly found in pyrite crystals and this is why this form is also called a pyrite hedron . The rhombic dodecahedron {110}, the trapezoidal {221} and the disdodecahedron (diploid) {321} are less common and mostly only in combination with other shapes . Curved, strip-shaped crystals are very rare.
Pseudomorphism from pyrite to brachiopod
Bulbous-spherical pyrite-limonite concretion (trade name Bojistein )
Epitaxies are also known, i.e. regular adhesions between pyrite and its orthorhombic relative, marcasite . In this form of intergrowth, two cube faces of pyrite are aligned parallel to a base and a prism face of marcasite.
Physical Properties

Due to its metallic sheen and its golden color, pyrite was and is often confused with gold . Unlike real gold, however, pyrite is not malleable and is much harder than the precious metal. In addition, pyrite leaves a distinct black line on the marking board (with an occasional greenish or bluish tinge), while gold leaves a gold-colored line. However, at some sites, pyrite can actually contain tiny amounts of gold that can make it an economically minable gold ore. Occasionally pyrite shows a brownish or variegated tarnish .
The Mohs hardness of 6 to 6.5 for pyrite, which is unusually high for a sulfide, is due to the high proportion of covalent bonds.
Pyrite shows only an indistinct cleavage after the cube faces {001}. Overall, however, it reacts brittle to mechanical stress and breaks like glass like a shell .
Chemical properties
Pyrite is soluble in nitric acid , concentrated hydrochloric acid and in hot concentrated sulfuric acid . The mineral decomposes when heated above 743 ° C. From a temperature of 570 ° C, pyrite turns into pyrrhotite .
In front of the soldering tube , pyrite burns with a bluish flame and gives off sulfur dioxide (SO 2 ). A black, magnetic ball is created as a melt product.
Electrical Properties
Pyrite is a natural semiconductor , the energy gap between the valence band and the conduction band is reduced by the incorporation of foreign atoms . Doping with arsenic leads to a p-semiconductor. The band gap is between 0.8 and 1.8 eV . It is still unclear whether these are direct or indirect strip edge transitions. An energy of 0.95 eV is usually specified for the indirect transition. The dielectric constant of pyrite is 20.8.
Due to their semiconducting properties, natural pieces of pyrite were previously used in detector receivers ( detector radio ) as crystal detectors for demodulation . By making contact by hand according to the principle of a tip diode , a needle was used to search for a region on the stone suitable as a diode. The fact that pyrite was still used militarily for wireless telegraphy during the First World War (until 1918) can be proven on the basis of a letter dated February 2, 1918 in the archive of the Mineralogical State Collection in Munich . The company Dr. F. Krantz. Rheinisches Mineralien-Kontor (owner at that time was Friedrich Krantz, a nephew of Adam August Krantz ) asks the then head of the Mineralogical State Collection to sell pyrite at a price of 12 marks per 100 g with the prospect of it after the end of the war It shouldn't be difficult to replace this pyrite.
Magnetic properties
In 2020, scientists at the University of Minnesota succeeded in transforming pyrite , which is not actually known for magnetism, into an iron magnet . For this purpose, the material was brought into contact with an electrolyte solution and then a weak voltage of one volt was applied. After the voltage was separated again, the pyrite returned to its non-magnetic original state.
Modifications and varieties
The compound FeS 2 is dimorphic , which means that it occurs in addition to the cubic crystallizing modification pyrite as an orthorhombic crystallizing modification marcasite .
The following pyrite varieties are known to date :
- The nickel-containing bravoite (also Mechernichite after its discovery on the Mechernich lead mountain near Mechernich , North Rhine-Westphalia) was first discovered and described in deposits of the Ragra Mine (Minasragra), Junín, Cerro de Pasco , Alcides Carrión Province, Pasco , Peru . It was named after the Peruvian scientist Jose J. Bravo (1874–1928).
- Hengleinite is a variety containing cobalt and nickel that was first described in rocks from Müsen , Siegerland , North Rhine-Westphalia .
- For the Arsenic "Gelpyrit" (also Melnikovitpyrit ) no exact formula can be indicated, as it consists of a gel-like mixture of FeS and FeS 2 crystallized. It then consists mainly of pyrite, but also contains small amounts of FeS and possibly additional water inclusions . Gel residues are also occasionally preserved.
Education and Locations
Like its less stable cousin marcasite and the lead sulfide mineral galena, pyrite is a so-called “runner” that is stable under igneous as well as sedimentary and metamorphic formation conditions. Due to its wide range of stability, pyrite is one of the most widespread minerals and by far the most abundant sulfide mineral that often forms massive pyrite deposits. So far (as of 2020), pyrite has been found at almost 43,000 sites worldwide.
Even if pyrite occurs in almost all types of deposits, the most important prerequisite for its actual formation is the extensive lack of oxygen . The divalent iron (Fe 2+ ) required in the chemical formula can only exist under oxygen-poor or oxygen-free conditions. This is also the reason for the main formation area of pyrite from hydrothermal solutions , since in this area the oxygen partial pressure is so low up to over 300 ° C (in an acidic environment) that iron is only present as Fe 2+ . Under the conditions in the oxygen-rich atmosphere on the earth's surface, pyrite cannot develop. Once formed, however, pyrite is metastable like diamond , albeit less stable than the latter.
As a pass-through mineral, pyrite can occur in association with many other types of mineral , although the sulfides and sulfosalts predominate. In addition to galena and marcasite, frequent education partners include arsenopyrite , chalcopyrite , pyrrhotite and sphalerite . In addition there are barite , calcite , fluorite , hematite and quartz
Igneous formation
In igneous rocks , pyrite is widespread as an accessory component, with the upper limit of formation being determined by the decomposition into pyrrhotite (Fe 1 − x S) and sulfur (S) depending on pressure and temperature . In intramagmatic nickel-pyrrhotite deposits , pyrite initially only forms to a minor extent and only occurs at lower temperatures. Thus pyrite found for example in the the South African Bushveld Complex belonging Merensky Reef in larger quantities. Furthermore, he in granite - pegmatites , found albeit in rather small quantities.
Hydrothermal formation
The main formation area of pyrite is of hydrothermal origin, whereby the mineral can occur in all hydrothermal vein and displacement deposits both in coarse masses as well as in perfectly formed crystals. Large displacement deposits were known in Tuscany near Gavorrano , Niccioleta and Boccheggiano . Another known massive sulphide displacement deposit is Madem-Lakkos about 3.5 km away from Stratoni and about 100 km southeast of Thessaloniki on the Greek peninsula of Chalkidiki .
A transition area is formed by submarine sedimentary-exhalative deposits , in which pyrite, along with other sulfides, is one of the most important ore minerals. For example, the mineral has been produced for around 25,000 years from salt solutions, some of which are more than 60 ° C, and collects together with other sulphides in deep basins of the Red Sea . Over time, massive, metal-rich layers of sulphide sludge formed there. The largest known deposit of this type is the “Atlantis II Deep” at a depth of around 2000 meters with an area of around 90 km², which is roughly the size of Manhattan . Of black smokers at the bottom of the deep sea is also known that they can emit large amounts of pyrite, which precipitates in the cold sea water.
Well-known deposits of this type also include the Rammelsberg mine in the Lower Saxony district of Goslar (Germany), the former Drei Kronen & Ehrt mine (formerly Grube Himmelsfürst or Grube Einheit ) near Elbingerode in the Harz district (Saxony-Anhalt) and the Hüttenberger Erzberg in Northeast of Carinthia in Austria.
Sedimentary formation
In sediments, pyrite is initially always precipitated as amorphous iron monosulfide (FeS) due to the metabolic activity of sulfate-reducing bacteria . Under anoxic conditions, for example in sea basins like the Black Sea , it can also precipitate directly, even in the water column. In principle, dissolved sulfate ions are always present in sufficient concentration in seawater. The process can also take place in the soil, provided that sulphate-rich groundwater is available, for example in the vicinity of gypsum deposits. The predominantly amorphous iron monosulphide reacts, including through microbially precipitated sulfur, to form iron disulphide (FeS 2 ), which crystallizes as pyrite or marcasite. Other iron sulfide phases such as mackinawit and greigit are also formed, but are usually not stable in the long term, but are further converted to pyrite by the hydrogen sulfide produced by the bacteria.
This reaction can take place directly in the water (with the formation of concentric rounded aggregates called framboids), in sediments mostly over the course of decades to centuries. Themodynamically (as shown in the Pourbaix diagram ), pyrite is the only stable phase under anoxic conditions in seawater. Marcasite can only be deposited in acidic water, i.e. in limnic sediments, but not in alkaline sea water. At least five million tons of pyrite are formed in this way each year, mainly in marine sediments.
During the diagenesis of the sediments, grain coarsening usually occurs due to collective crystallization, whereby pyrite has the ability to displace almost all rock-forming minerals occurring in sediments and thus to create space. A preferred location for the deposition of pyrite are cavity structures, such as within mollusc shells (ammonite housings and the like) embedded in the sediment. The processes outlined above can accumulate so much pyrite here over long periods of time that the chambers of the ammonite housings are sometimes completely filled and a stone core is formed from pyrite. With more compression, pyrite can grow into larger crystals and even replace clamshells or bones. In this way, fossils can be completely transformed. An example of this are the ammonites of the Jura of the Franconian Alb and Württemberg , known as "gold snails" (also known locally as gold snails ) .
Finally pyrite also comes in brown and black coal as well as in oxygen-free aquifers before. In this environment it is usually poorly crystallized and very sensitive to oxidation.
Metamorphic Education

Under the influence of regional metamorphic forces , pyrite can be preserved into the kata zone (over 500 ° C), where it is finally converted into pyrrhotite. However, already begins at significantly lower temperatures in the Epizone a re- and grain growth, grow in the large of tiny, distributed in the rock pyrite grains crystals. This form of crystal formation in the solid state is very often found in gneiss and green slate (also chlorite slate ). Well-known sites of this type were and are among others the emerald deposit in the Habach Valley in the Hohe Tauern in the Salzburg region (Austria) with finds of centimeter-sized pyrite cubes as well as the formerly economically important pyrite ore deposit, the Bayerland mine in the Upper Palatinate municipality of Leonberg (Bavaria) and The Lengenbach mine in the Binn valley in the Swiss canton of Valais is one of the most famous, because it is richest in minerals .
Until the beginning of the 1980s, the pyritic sulfur mining near Meggen in the Sauerland (North Rhine-Westphalia) was one of the economically important deposits in Germany with an annual production of around 450,000 t of pyrite.
Other well-known localities of this type include the former fluorite - pit Hohewarte in Gernrode Saxony-Anhalt (Germany) with hornfels - and Skarngesteinen where next dezimetergroßen pyrite crystals and aggregates and the rare mineral Cronstedtit occurs, for Ankogelgroup belonging Plattenkogel with his aplitic gneiss in Carinthia on the border with Salzburg and the gneiss and mica schist of the Schwarzkopf near Bad Gastein in the Salzburg district of St. Johann im Pongau in Austria, where up to 9 cm large pyrite crystals could be recovered
Well-known iron and copper deposits of the Skarn type are the Trepča Stan Terg Mine in the Trepča complex northeast of Mitrovica in Kosovo with several centimeter pyrite crystals and aggregates as well as pseudomorphoses according to Pyrrhotin , the Nikolaevskiy mine near Dalnegorsk in the Far East of Russia in general very well developed sulphide mineral grades ( arsenopyrite , chalcopyrite , galena , pyrite, pyrrhotite, sphalerite ) as well as the Fengjiashan mine near Daye in the Chinese province of Hubei, where pyrite occurs mostly in association with quartz in crystal aggregates, some of which are decimeter-sized.
Important pyrite finds


The largest pyrite cubes with an edge length of up to 50 cm were mined in the mines on Halkidiki in Greece. Up to 30 cm large pyrites are known from the Climax ore deposit (mainly molybdenite, but also cassiterite, hebnerite and pyrite) near the town of the same name in Lake County in the US state of Colorado. Up to 35 kg and up to 22 cm large crystals occurred in the gold deposit Beryozovsky (English Berezovsk ) in the Russian Sverdlovsk Oblast to light. Well-crystallized pyrite with a diameter of up to 20 cm occurred in Rio Marina on the island of Elba in Italy and in the Sámo mine near Hnúšťa in Slovakia. At least up to 15 cm large crystals were found in Huallanca (Huánuco) and Santiago de Chuco in Peru , although exceptional finds are always possible.
The Cakmakkaya copper ore mine near Murgul ( Göktaş until 1987 ) in Turkey produces pyrite crystals that are not as large as they are, but extremely rich in shape. In addition to octahedra several centimeters in size, there were also combinations of pentagon dodecahedra ( pyritoedron ) and icosahedron . These pyrites were created in a combination of volcanic sedimentary and hydrothermal ore deposits. Side rocks of the veins are limestone as well as rhyolite , trachytic , andesitic and basaltic breccias .
Celebrity have also called the "Iron Cross" Crystal twins , two after pyrite law deformed pentagonal dodecahedron, from the Weserbergland , around Vlotho and in Extertal in East Westphalia, Germany. Also known is Ross County in the US state of Ohio with its sometimes bizarre pyrite concretions , the so-called "pyrite snakes".
The mines of Riotinto in the pyrite belt of the South Iberian peninsula (also Rio Tinto , Spanish Faja Pirítica Ibérica ), where the pyrite is present in the form of fine-grained deposits with an estimated mass of around one billion tons, are among the largest sedimentary-exhalative deposits and oldest mining areas in Spain , as well as the place Navajún ( La Rioja ), also located in Spain, with the most finds of perfect and high-gloss pyrite cubes worldwide. Crystal groups can be up to 30 cm long and the largest cubes can have an edge length of up to 6 cm (according to other sources also up to 8 cm).
Other known find areas
In addition to the exemplified storage and discovery sites of the various educational types, the following sites were and are known due to extraordinary pyrite finds:
- France
- La Lande quarry near Plumelin in Brittany
- Aigue Bonne quarry near Saint-Raphaël on the Côte d'Azur in the Var department
- Ireland
- The Ballygown South , Ballynoe and Mogul Silver Mines in the District of Silvermines , County Tipperary
- Canada
- Nanisivik on Baffin Island in the Nunavut territory with the silver-rich lead and zinc mine of the same name, where very well-formed marcasite and pyrite crystals, complex crystal combinations as well as twins based on the Iron Cross, pseudomorphoses based on marcasite and epitaxial adhesions of the two were found.
- Elizabethtown-Kitley in Leeds and Grenville United Counties in Ontario with the Shipman iron mine (also Billings Mine ) and pyrite finds up to 9 cm in size
- The now abandoned town of Elsa in the Yukon Territory, where mainly silver, lead and zinc were mined, but also well-developed pyrites and marcasites were found next to unusual, partly colored tarnished polybasite and stephanite crystals
- Norway
- The municipality of Evje og Hornnes with several feldspar quarries and well-formed, centimeter-sized pyrite cubes
- United States
- The Zn-Pb-Ag-Cu-Au-Mn mine New Jersey Zinc Eagle Mine ( Eagle Mine or Gilman Mine for short ) near Gilman in Eagle County of Colorado with pyrite stages over 20 cm in size
- The Roxbury Iron Mine in Connecticut's Litchfield County is primarily known as the largest siderite deposit in North America, but it also provides excellent pyrite crystals
- Others
Pyrite was also found in rock samples from the hydrothermal fields of the Mid-Atlantic Ridge , the Central Indian Ridge , the East Pacific Ridge (Sea of China, Japan and Okhotsk) as well as outside the Earth on the moon in Mare Crisium , the landing area of the Luna-24 mission .
weathering
In an oxygen-rich environment e.g. B. in the oxidation zone of sulphide deposits, pyrite is exposed to weathering and therefore transforms slowly. First of all, the sulfur is converted into (SO 4 ) 2− by oxidation of S 2− and iron sulphates such as melanterite ( iron vitriol ) or copiapite are formed . When the sulfur is removed in solutions, the trivalent iron remains and with oxygen forms oxides and hydroxides such as limonite (a mixture of mainly goethite and lepidocrocite ) and finally hematite through dehydration of limonite.
With a correspondingly slow conversion, pseudomorphoses from goethite or limonite to pyrite arise in this way, which may still contain residues of pyrite in the core. However, the oxidation of pyrite releases so much energy that it can self-ignite, especially when it is fine-grained, and cause mine fires . Among other things, a fire of coal seams near Ravat on the Jaghnob River (also Yagnob ) in Tajikistan was triggered, which has been raging for over 2000 years ( i.e. already at the time of Alexander the Great ).
Pyrite in domestic or museum collections also disintegrates over time under the influence of atmospheric oxygen and humidity. The so-called "pyrite disease " begins with efflorescence (see also mineral aggregate , crusts, efflorescence), which creates cracks along which the samples crumble and disintegrate. The most stable are well-formed crystals and steps with smooth crystal surfaces, some of which can last for centuries. Surface treatment can also slow down the weathering-related decay. In contrast, excessively high humidity has an accelerating effect.
Goethite to pyrite pseudomorphism from Pelican Point quarry , Utah Lake , USA
Cannelkohle (also Kannel or Kännelkohle) with embedded pyrite with "pyrite disease" (white powder on the remains of the golden-yellow pyrite is iron sulfate)
Biological importance
According to a theory of the biochemist Günter Wächtershäuser , the beginning of the chemical processes necessary for life may have originated on pyrite. Life arose under anaerobic conditions like those necessary for the formation of pyrite. Pyrite offers several prerequisites for starting and maintaining the simplest chemical metabolic processes. On the one hand, the positive charge on the crystal surfaces of pyrite has a beneficial effect on the cohesion of the predominantly negatively charged building blocks for the synthesis of organic molecules. On the other hand, the pyrite growth provides enough energy and dynamics to drive and maintain synthesis reactions of biomolecules (see also Chemical Evolution # Iron-Sulfur-World (ESW) ).
According to Wächtershäuser, the fact that iron and sulfur still play an important role in numerous biochemical processes can serve as an indication of this possibility of the development of life processes; basically z. B. as iron-sulfur clusters in enzymes of anaerobic life forms.
use
As a raw material
Besides elemental sulfur, pyrite is the most important sulfur raw material for the production of sulfuric acid . By means of oxidizing roasting , the iron sulphide is first converted into iron (III) oxide and sulfur dioxide with the help of oxygen . The latter is further processed into sulfur trioxide and finally sulfuric acid.
The iron (III) oxide (also purple ore or gravel burn , Fe 2 O 3 ) left over from sulfuric acid production is processed into iron in blast furnaces . Gravel burn-off is also used as a polishing agent and paint base. A well-known polishing agent was the polishing red produced in Bodenmais in the Bavarian Forest by carefully oxidizing pyrite and pyrrhotite .
In 1999, only around three million tons of pyrite were roasted for sulfuric acid production in Europe; the greater part of sulfuric acid is now obtained from the desulfurization of fossil fuels and other exhaust gases.
Historically, pyrite and marcasite were used to extract vitriol . These were on heaps of weathering exposure - the pyrite had to advance some of the sulfur using are expelled from special "sulfur furnaces" while marcasite by itself weathered (hence its synonym Vitriolkies ) - and converted thereby slowly in vitriol (here: green vitriol ) around. The seepage water was collected and the vitriol it contained was leached out .
In addition to the production of vitriol, pyrite was also used to extract alum , i.e. obtained from roasted and leached, pyrite-containing alum slate . During production, pyrite formed the basis of the sulfuric acid required for the formation of aluminum sulfate, which was processed into potassium alum together with potassium sulfate.
With local enrichment with copper z. B. by adding chalcopyrite , pyrite is also obtained as copper ore and by adding gold as gold ore.
In the manufacture of high quality steel such as stainless steel , pyrite is added to improve machinability. The reason for this is the property of sulfur to form a sulfur matrix in steel.
In 2015, researchers at Empa and ETH were looking for an inexpensive alternative to lithium-ion batteries and developed what is known as a “fool’s gold battery” in which the anode (positive pole) is made of magnesium and the cathode (negative pole) is made of pyrite. The associated electrolyte consists of magnesium and sodium ions . The advantages of this new battery, in addition to the considerably lower raw material prices of magnesium (15 times cheaper) and pyrite compared to lithium , are that it cannot explode because magnesium is safer as an anode than lithium. In addition, according to previous research results, the fool's gold battery seems to have a longer service life, as it hardly lost any of its performance even after 40 charging and discharging cycles. The only disadvantage is the overall performance, which is still rather low, which has so far excluded its use in mobile devices and electric cars.
As a gem and collector's item
Pyrite belongs to the group of funeral jewelry , but is also worn on other occasions and made into gemstones, usually in a natural form as a pendant or brooch , but also cut as a ring stone or on necklaces . The Maya pyrite was in the 9th century also next to Jade , cinnabar , hematite , quartz , serpentine and turquoise a popular dental jewelery were drilled for precisely drilled holes in the front teeth.
However, pyrite is not particularly suitable as a wearing jewelry because it is sensitive to the effects of heat, which causes problems even when grasping. Due to its sensitivity to acids, the crystal surfaces “go blind” over time. Since pyrite is very similar to marcasite , it is often sold under this incorrect name. However, marcasite is even more sensitive and disintegrates after a few years.
As a collector's item, pyrites are in great demand as well-formed crystals and steps, as well as pyritized fossils. Famous sites here were and are among others Elba in Italy for up to 15 cm large, sharp-edged and high-gloss pyritohedra, Central Peru ( Cerro de Pasco , Chungar and others) for sometimes extremely heavy crystal steps with up to 10 cm large cubes and combinations, Navajún in northern Spain for the world's most and most perfect cubes as well as crystal groups up to 30 cm in size, Stratoni on the Greek peninsula of Chalkidiki with high-gloss steps, cubes, octahedra and pyritohedra and frequent giant crystal formation with an edge length of up to 50 cm as well as the Saxon and Bohemian Ore Mountains for several centimeters in size Pseudomorphoses of pyrite and marcasite after pyrrhotite .
Significance for the environment
The pyrite and other sulfur compounds contained in lignite and hard coal release the sulfur contained in the combustion process as sulfur dioxide (SO 2 ) to the flue gases. When this gas gets into the atmosphere, sulphurous acid forms in water droplets , which contributes significantly to the formation of “acid rain”. The sulfur dioxide can largely be retained by measures to remove flue gas .
The pyrite contained in the groundwater layers can also be oxidized in the presence of oxygen. This oxidation is mainly catalyzed by bacteria . The oxidation of pyrite by denitrifying , iron and sulfur oxidizing bacteria with nitrate as an indirect oxidizing agent is of great importance . This is a process consisting of several abiotic and bacterial redox reactions in which the sulfide sulfur of the pyrite is ultimately oxidized to sulfate (SO 4 2− ) and nitrate is reduced to elemental, molecular nitrogen (N 2 ). This process is called “ denitrification ” by pyrite. In this way, 1,000 tons of pyrite are converted annually in the water catchment areas of Stadtwerke Hannover AG.
Since, according to the Drinking Water Ordinance, the limit value for nitrate at 50 mg / l is lower than that for sulfate at 240 mg / l, denitrification by pyrite means a relief in terms of compliance with the nitrate limit value. The iron contained in the pyrite and other metallic accompanying elements such as manganese or nickel can partially pass into the water and must be eliminated during the drinking water treatment .
The pyrite contained in lignite is one of the sources of acidification of residual holes .
Esoteric
Pyrite is used by esotericists as a healing stone against arthritis and sciatic pain . Pyrite grown in radial directions - a so-called "pyrite sun" - is said to be worn as an amulet pendant on the neck on the one hand against stomach and digestive disorders and on the other hand to strengthen the immune system. However, there is no scientific evidence of its effectiveness.
See also
literature
Monographs:
- Peter Bayliss: Crystal structure refinement of a weakly anisotropic pyrite . In: American Mineralogist . tape 62 , 1977, pp. 1168–1172 (English, rruff.info [PDF; 593 kB ; accessed on May 3, 2020]).
- Christa Behmenburg, Günter Grundmann, Rupert Hochleitner, Peter Huber, Peter Kolesar, Franziska von Kracht, Helmut Mayr, John Medici, Hans Jörg Müllenmeister, Erich Offermann, Ermengildo Pini, Köbi Siber, Günter Wächtershäuser, Stefan Weiß: pyrite and marcasite. The iron everywhere mineral (= Christian Weise [Hrsg.]: ExtraLapis . Volume 11 ). Weise, Munich 1996, ISBN 3-921656-38-9 .
- David Rickard : Pyrite: A Natural History of Fool's Gold . Oxford University Press, New York 2015, ISBN 978-0-19-020367-2 .
Compendia:
- Helmut Schrätze , Karl-Ludwig Weiner : Mineralogy. A textbook on a systematic basis . de Gruyter, Berlin; New York 1981, ISBN 3-11-006823-0 , pp. 245-251 .
- Hans Jürgen Rösler : Textbook of Mineralogy . 4th, revised and expanded edition. German publishing house for basic industry (VEB), Leipzig 1987, ISBN 3-342-00288-3 , p. 66, 87, 322-327 (p. 66: halite structure type ; p. 87: pyrite law ; p. 322-327: pyrite-marcasite group ).
- Paul Ramdohr : The ore minerals and their adhesions . 4th, revised and expanded edition. Akademie-Verlag, Berlin 1975, p. 848-863 .
Web links
- Mineral Atlas: Pyrite and Mineral Portrait Pyrite (Wiki)
- Pyrite. In: mindat.org. Hudson Institute of Mineralogy, accessed February 26, 2019 .
- Pyrite search results. In: rruff.info. Database of Raman spectroscopy, X-ray diffraction and chemistry of minerals (RRUFF), accessed on May 3, 2020 .
- American-Mineralogist-Crystal-Structure-Database - Pyrite. In: rruff.geo.arizona.edu. Retrieved May 3, 2020 .
- Thomas Seilnacht: Pyrite profile. In: seilnacht.com. March 28, 2020, accessed May 3, 2020 .
Individual evidence
- ^ A b c Hugo Strunz , Ernest H. Nickel : Strunz Mineralogical Tables. Chemical-structural Mineral Classification System . 9th edition. E. Schweizerbart'sche Verlagbuchhandlung (Nägele and Obermiller), Stuttgart 2001, ISBN 3-510-65188-X , p. 103 .
- ^ A b David Barthelmy: Pyrite Mineral Data. In: webmineral.com. Retrieved May 3, 2020 .
- ↑ a b Jürgen Oertel: Material-chemical and electronic investigations of cobalt-doped MOCVD layers made of pyrite for photovoltaic applications . Freie Universität Berlin, Berlin 2003 ( short description available online at refubium.fu-berlin.de - dissertation).
- ↑ a b c Hans Lüschen: The names of the stones. The mineral kingdom in the mirror of language . 2nd Edition. Ott, Thun 1979, ISBN 3-7225-6265-1 , p. 297 .
- ↑ Jürgen Weiner, Harald Floss: A sulfur gravel bulb from the Aurignacien vom Vogelherd, Baden-Württemberg - To the beginnings of fire production in the European Paleolithic . In: Archaeological Information . tape 27 , no. 1 , 2004, p. 59–78 ( online at Heidelberg University [accessed June 12, 2020]).
- ^ Bertrand Roussel: La production du feu par percussion de la pierre - Prehistoire, ethnography, experimentation . In: Préhistoires . tape 11 , 2005 (French).
- ↑ Sulfur pebbles in a prehistoric context at steinzeitwissen.de ( Memento from June 20, 2017 in the Internet Archive )
- ↑ a b Christa Behmenburg: Pyrites, Marcasita and Kieß . In: pyrite and marcasite. The iron everywhere mineral (= Christian Weise [Hrsg.]: ExtraLapis . Volume 11 ). Weise, Munich 1996, ISBN 3-921656-38-9 , p. 3 .
- ↑ Wolfgang Pfeifer (Ed.): Etymological dictionary of German . Licensed edition for ed. Kramer. Akademie-Verlag, Berlin 2012, ISBN 978-3-941960-03-9 , pp. 638 .
- ^ Wilhelm von Haidinger : Handbook of determining mineralogy, containing the terminology, systematics, nomenclature and characteristics of the natural history of the mineral kingdom . Braumüller & Seidel, Vienna 1845, p. 443 ( limited preview in Google Book search).
- ↑ a b Malcolm Back, William D. Birch, Michel Blondieau and others: The New IMA List of Minerals - A Work in Progress - Updated: March 2020. (PDF; 2.44 MB) In: cnmnc.main.jp. IMA / CNMNC, Marco Pasero, March 2020, accessed May 3, 2020 .
- ↑ Stefan Weiß: The large Lapis mineral directory. All minerals from A - Z and their properties. Status 03/2018 . 7th, completely revised and expanded edition. Weise, Munich 2018, ISBN 978-3-921656-83-9 .
- ↑ Ernest H. Nickel, Monte C. Nichols: IMA / CNMNC List of Minerals 2009. (PDF; 1.82 MB) In: cnmnc.main.jp. IMA / CNMNC, January 2009, accessed May 3, 2020 .
- ↑ Pyrite. In: Mineralienatlas Lexikon. Stefan Schorn u. a., accessed on May 5, 2020 .
- ↑ a b c d Pyrite . In: John W. Anthony, Richard A. Bideaux, Kenneth W. Bladh, Monte C. Nichols (Eds.): Handbook of Mineralogy, Mineralogical Society of America . 2001 (English, handbookofmineralogy.org [PDF; 63 kB ; accessed on May 5, 2020]).
- ↑ Hans Jürgen Rösler : Textbook of Mineralogy . 4th revised and expanded edition. German publishing house for basic industry (VEB), Leipzig 1987, ISBN 3-342-00288-3 , p. 322 .
- ↑ a b c d Friedrich Klockmann : Klockmann's textbook of mineralogy . Ed .: Paul Ramdohr , Hugo Strunz . 16th edition. Enke, Stuttgart 1978, ISBN 3-432-82986-8 , pp. 457 (first edition: 1891).
- ^ Helmut Schrätze , Karl-Ludwig Weiner : Mineralogie. A textbook on a systematic basis . de Gruyter, Berlin; New York 1981, ISBN 3-11-006823-0 , pp. 246 .
- ↑ Paul Ramdohr : The ore minerals and their adhesions . 4th, revised and expanded edition. Akademie-Verlag, Berlin 1975, p. 853 .
- ^ LA Clark, G. Kullerud: The sulfur-rich portion of the Fe-Ni-S system . In: Economic Geology . tape 58 , no. 6 , 1963, pp. 853-885 , doi : 10.2113 / gsecongeo.58.6.853 (English).
- ↑ Entry on pyrite. In: Römpp Online . Georg Thieme Verlag, accessed on July 9, 2011.
- ↑ Rupert Hochleitner: What is pyrite? What is marcasite? In: pyrite and marcasite. The iron everywhere mineral (= Christian Weise [Hrsg.]: ExtraLapis . Volume 11 ). Weise, Munich 1996, ISBN 3-921656-38-9 , p. 10-11 .
- ^ A b Helmut Schrätze , Karl-Ludwig Weiner : Mineralogie. A textbook on a systematic basis . de Gruyter, Berlin; New York 1981, ISBN 3-11-006823-0 , pp. 245 .
- ↑ a b c Martin Okrusch, Siegfried Matthes: Mineralogie. An introduction to special mineralogy, petrology and geology . 7th, completely revised and updated edition. Springer, Berlin [a. a.] 2005, ISBN 3-540-23812-3 , pp. 38-39 .
- ↑ a b c d Hans Jürgen Rösler : Textbook of Mineralogy . 4th revised and expanded edition. German publishing house for basic industry (VEB), Leipzig 1987, ISBN 3-342-00288-3 , p. 323 .
- ↑ Roger Blachnik (Ed.): Jean D 'Ans: Pocket book for chemists and physicists . 4th, revised and revised edition volume 3 . Springer, Berlin 1998, ISBN 3-540-60035-3 .
- ↑ Peter Möller: High-tech in nature - gold enrichment in pyrite and arsenopyrite . In: AGF annual magazine . 1994, p. 36 ( available online at gfz-potsdam.de [PDF; 1.1 MB ; accessed on February 26, 2019]).
- ^ Rupert Hochleitner: The pyrite radio . In: pyrite and marcasite. The iron everywhere mineral (= Christian Weise [Hrsg.]: ExtraLapis . Volume 11 ). Weise, Munich 1996, ISBN 3-921656-38-9 , p. 92 .
- ^ Voltage-induced ferromagnetism in a diamagnet. In: Science Advances. American Association for the Advancement of Science, accessed July 31, 2020 .
- ^ Helmut Schrätze , Karl-Ludwig Weiner : Mineralogie. A textbook on a systematic basis . de Gruyter, Berlin; New York 1981, ISBN 3-11-006823-0 , pp. 252 .
- ↑ Bravoite. In: mindat.org. Hudson Institute of Mineralogy, accessed February 26, 2019 .
- ↑ Hengleinite. In: mindat.org. Hudson Institute of Mineralogy, accessed February 26, 2019 .
- ↑ Paul Ramdohr : The ore minerals and their adhesions . 4th, revised and expanded edition. Akademie-Verlag, Berlin 1975, p. 863-864 .
- ↑ a b c d Helmut Schrätze , Karl-Ludwig Weiner : Mineralogie. A textbook on a systematic basis . de Gruyter, Berlin; New York 1981, ISBN 3-11-006823-0 , pp. 247-249 .
- ↑ Localities for Pyrite. In: mindat.org. Hudson Institute of Mineralogy, accessed February 26, 2019 .
- ↑ a b c Rupert Hochleitner: Mineral from another world. About the formation of pyrite deposits . In: pyrite and marcasite. The iron everywhere mineral (= sage [Hrsg.]: ExtraLapis . Volume 11 ). Weise, Munich 1996, ISBN 3-921656-38-9 , p. 16-17 .
- ↑ a b List of locations for pyrite in the Mineralienatlas and Mindat , accessed on June 16, 2020.
- ^ Metal sludge in the Red Sea. In: worldoceanreview.com. World Ocean Review , accessed June 3, 2020 .
- ↑ Pit unit (three crowns and honor). Description of the location, history and mineral finds. In: Mineralienatlas Lexikon. Stefan Schorn u. a., accessed on June 3, 2020 .
- ↑ Julia Kirchner: How is pyrite formed in marine sediments? In: uol.de. University of Oldenburg , November 7, 2019, accessed June 7, 2020 .
- ↑ Martin AA Schoonen: Mechanisms of sedimentary pyrite formation . In: JP Amend, KJ Edwards, TW Lyons (Eds.): Sulfur biogeochemistry - Past and present. Geological Society of America Special Papers . tape 379 , 2004, pp. 117–134 , doi : 10.1130 / 0-8137-2379-5.117 (English, available online at academia.edu [accessed June 3, 2020]).
- ↑ Manuela Schünge, Ilka Ottleben: Ur-Energie: The metabolism of all metabolism? In: laborpraxis.vogel.de. March 21, 2019, accessed June 7, 2020 .
- Jump up ↑ Joana Thiel, James M. Byrne, Andreas Kappler, Bernhard Schink, Michael Pester: Pyrite formation from FeS and H 2 S is mediated through microbial redox activity . In: Proceedings of the National Academy of Sciences of the United States of America (PNAS) . tape 116 , no. 4 , 2019, p. 6897–6902 , doi : 10.1073 / pnas.1814412116 (English, pnas.org [PDF; 1,3 MB ; accessed on June 7, 2020]).
- ↑ Rupert Hochleitner: Mineral from another world. About the formation of pyrite deposits . In: pyrite and marcasite. The iron everywhere mineral (= Christian Weise [Hrsg.]: ExtraLapis . Volume 11 ). Weise, Munich 1996, ISBN 3-921656-38-9 , p. 21 .
- ↑ Meggen mine, Sauerland. Description of the location, history and mineral finds. In: Mineralienatlas Lexikon. Stefan Schorn u. a., accessed on June 3, 2020 .
- ↑ Type locality Hohe Warte Mine, Hagental, Gernrode, Harz, Saxony-Anhalt, Germany. In: mindat.org. Hudson Institute of Mineralogy, accessed June 3, 2020 .
- ↑ Examples of images of pyrite from Schwarzkopf, Bad Gastein, St. Johann im Pongau, Salzburg, Austria. In: mindat.org. Hudson Institute of Mineralogy, accessed June 3, 2020 .
- ↑ Photo examples of pyrite from the Trepča Stan Terg mine, Trepča complex, Mitrovica, Kosovo. In: mindat.org. Hudson Institute of Mineralogy, accessed June 3, 2020 .
- ^ Nikolaevskiy Mine, Dalnegorsk, Far East, Russia. Description of the site and mineral finds. In: Mineralienatlas Lexikon. Stefan Schorn u. a., accessed on June 3, 2020 .
- ↑ Examples of pictures from the Fengjiashan Mine, Daye, Hubei, China. In: mindat.org. Hudson Institute of Mineralogy, accessed June 3, 2020 .
- ↑ a b c d Günter Grundmann: The top ten pyrite sites . In: pyrite and marcasite. The iron everywhere mineral (= Christian Weise [Hrsg.]: ExtraLapis . Volume 11 ). Weise, Munich 1996, ISBN 3-921656-38-9 , p. 32-37 .
- ↑ Climax Mine. Description of the location, history and mineral finds. In: Mineralienatlas Lexikon. Stefan Schorn u. a., accessed on June 3, 2020 .
- ↑ Mineral portrait pyrite: The largest crystals. In: Mineralienatlas Lexikon. Stefan Schorn u. a., accessed on June 3, 2020 .
- ↑ a b c Petr Korbel, Milan Novák: Mineral Encyclopedia (= Dörfler Natur ). Edition Dörfler im Nebel-Verlag, Eggolsheim 2002, ISBN 978-3-89555-076-8 , p. 42 .
- ↑ Examples of images from the Cakmakkaya mine, Murgul Cu-Zn-Pb deposit, Murgul, Artvin, Turkey. In: mindat.org. Hudson Institute of Mineralogy, accessed June 3, 2020 .
- ^ The mines of Riotinto / Minas del Rio Tinto. Description of the location, history and mineral finds. In: Mineralienatlas Lexikon. Stefan Schorn u. a., accessed on June 17, 2020 .
- ↑ Klaus Schilling: Pyrite: Twin or Epitaxy? In: Kristall2000.de. November 17, 2019, accessed June 3, 2020 .
- ↑ Photo examples of pyrite from the Shipman Mine (Billings mine), Elizabethtown, Leeds and Grenville Counties, Ontario, Canada. In: mindat.org. Hudson Institute of Mineralogy, accessed June 3, 2020 .
- ↑ Type locality Roxbury Iron Mine (Shepaug Iron Company Mine; Shepaug Spathic Iron and Steel Company Mine), Mine Hill (Ore Hill), Roxbury, Litchfield County, Connecticut, USA. In: mindat.org. Hudson Institute of Mineralogy, accessed June 3, 2020 .
- ^ Type locality Mid-Atlantic Ridge complex, Atlantic Ocean. In: mindat.org. Hudson Institute of Mineralogy, accessed June 3, 2020 .
- ↑ Type locality Central Indian Ridge, Indian Ocean. In: mindat.org. Hudson Institute of Mineralogy, accessed June 3, 2020 .
- ↑ Type locality East Pacific Rise, Pacific Ocean. In: mindat.org. Hudson Institute of Mineralogy, accessed June 3, 2020 .
- ↑ Luna 24 landing zone. Description of the site and mineral finds. In: Mineralienatlas Lexikon. Stefan Schorn u. a., accessed on June 3, 2020 .
- ↑ Hans Jürgen Rösler : Textbook of Mineralogy . 4th revised and expanded edition. German publishing house for basic industry (VEB), Leipzig 1987, ISBN 3-342-00288-3 , p. 384 .
- ↑ Victor Victorovich Sharygin, Ella Sokol, Dmitriy Belakovskii: Fayalite-sekaninaite paralava from the Ravat coal fire (central Tajikistan) . In: Russian Geology and Geophysics . tape 50 , no. 8 , August 2009, p. 703–721 , doi : 10.1016 / j.rgg.2009.01.001 (English, available online for download from researchgate.net [accessed June 3, 2020]).
- ↑ Maximilian Glas: Did life arise on pyrite? In: pyrite and marcasite. The iron everywhere mineral (= Christian Weise [Hrsg.]: ExtraLapis . Volume 11 ). Weise, Munich 1996, ISBN 3-921656-38-9 , p. 29 (Interview with the Munich chemist and patent attorney Günter Wächtershäuser).
- ^ A b Helmut Schrätze , Karl-Ludwig Weiner : Mineralogie. A textbook on a systematic basis . de Gruyter, Berlin; New York 1981, ISBN 3-11-006823-0 , pp. 251 .
- ^ ER Riegel, James A. Kent: Riegel's Handbook of Industrial Chemistry . Springer, 2003, ISBN 0-306-47411-5 , pp. 503 (English).
- ↑ Ernst Ludwig Schubarth: Elements of technical chemistry: for use in teaching in the Königl. Industrial Institute and the Provincial Industrial Schools of the Prussian State . 1st volume, 2nd section. Rücker, Berlin 1832, p. 131 ( limited preview in Google Book Search [accessed June 7, 2020]).
- ↑ 2000087468X # Vitriol Vitriol. In: Brockhaus Bilder-Conversations-Lexikon , Volume 4. Leipzig 1841, pp. 613–614
- ↑ Pyrite / pebbles - product description. In: coftech.de. Retrieved June 3, 2020 .
- ↑ Angelika Jacobs: Inexpensive fool's gold battery. In: nzz.ch. Neue Zürcher Zeitung, November 13, 2015, accessed on June 3, 2020 .
- ^ A b Leopold Rössler: Gemstone Knigge - Pyrite. BeyArs.com, accessed May 3, 2020 .
- ^ Joel B. Schilling: Mayan Dentistry. In: jbschilling.com. May 9, 2005, accessed June 20, 2020 .
- ^ A b Walter Schumann: Precious stones and gemstones. All kinds and varieties. 1900 unique pieces . 16th, revised edition. BLV Verlag, Munich 2014, ISBN 978-3-8354-1171-5 , pp. 290, 292 .
- ↑ Georg Rüschemeyer: As beautiful as a bath in vinegar and sulfur. The coal mining in the open pit leaves a lunar landscape with many water holes. But instead of the dreamy bathing lakes for tourists, extreme waters are created in some places. In: faz.net. Frankfurter Allgemeine (FAZ), June 18, 2006, accessed on February 26, 2019 .