Metastability
Metastability is a weak form of stability. A metastable state is stable to small changes but unstable to larger changes.

An example of this is the system of wood and atmospheric oxygen at room temperature: From a thermodynamic point of view, the spontaneous combustion of the carbon chemically bound in it with the oxygen to carbon dioxide would lead to a more stable state. Without activation, i.e. a sufficiently large supply of energy such as igniting the wood, this will not happen.
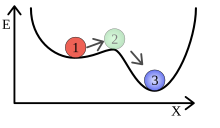
This is clearly shown in the picture on the right: A ball lies in a small hollow on a mountain slope. As long as the ball is only slightly deflected in the trough, it rolls back to its deepest point. This represents a local minimum. If it is deflected more strongly, it can roll down the mountainside and reach the global minimum. So first a certain minimum energy has to be applied before the state of the system changes.
Metastable phases have a higher energy (more correct: free enthalpy - under defined conditions such as constant pressure and constant temperature) than the stable phase. Due to a high activation energy , they do not or only slowly convert into the stable phase. This energetic basic principle of metastable states can also be used as a method for energy storage , as it also happens in principle, for example, in storage power plants .
An example of a metastable phase is diamond , which should spontaneously transform into graphite at atmospheric pressure ; however, the speed of this process is negligible at room temperature. Another example is the tin plague : The metallic phase of tin becomes metastable below 13 ° C and slowly transforms into the non-metallic phase, which is more stable at these temperatures. Further examples are supercooled water, glass (the most stable state would be the crystalline silicates) and oversaturated solutions, which are used in hand warmers , for example .
See also
Individual evidence
- ^ CHP Lupis: Chemical Thermodynamics of Materials , Elsevier, Amsterdam, 1983, ISBN 0-444-00713-X .
- ↑ Future concept of electrochemical energy storage. Website of the Energy Storage Initiative - Research Initiative of the Federal Government , May 2, 2016, accessed on November 1, 2017.
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1005.