Chalcopyrite
| Chalcopyrite | |
|---|---|
| Chalcopyrite (golden yellow, partially tarnished with bright colors ), galena (gray) and quartz (colorless) from the Borieva mine, Madan Erzfeld, Bulgaria (size: 7.4 cm × 6.4 cm × 4.1 cm) | |
| General and classification | |
| other names |
|
| chemical formula | CuFeS 2 |
|
Mineral class (and possibly department) |
Sulfides and sulfosalts |
|
System no. to Strunz and to Dana |
2.CB.10a ( 8th edition : II / B.02) 02.09.01.01 |
| Similar minerals | Pyrite , marcasite , magnetic gravel , gold |
| Crystallographic Data | |
| Crystal system | tetragonal |
| Crystal class ; symbol | tetragonal-scalenohedral; 4 2 m |
| Space group | I 4 2 d (No. 122) |
| Lattice parameters | a = 5.29 Å ; c = 10.42 Å |
| Formula units | Z = 4 |
| Twinning | more common than single crystals, according to {112} and {012} interpenetrating or cyclic twins |
| Physical Properties | |
| Mohs hardness | 3.5 to 4 |
| Density (g / cm 3 ) | measured: 4.1 to 4.3; calculated: 4.283 ( VHN 100 = 187 to 203 basal or 181 to 192 vertical) |
| Cleavage | indistinct after {011} and {111} |
| Break ; Tenacity | shell-like, uneven; brittle |
| colour | golden to brass yellow; after a while starting up colorful |
| Line color | greenish black to black |
| transparency | opaque |
| shine | Metallic luster |
Chalcopyrite , also known as copper pyrites , yellow pebbles , pyrites aureo colore or geelkis , is a very common mineral from the mineral class of " sulfides and sulfosalts " with the chemical formula CuFeS 2 . The compound therefore consists of one part each of copper and iron and two parts of sulfur and is therefore, chemically speaking, a copper-iron sulfide.
Chalcopyrite crystallizes in the tetragonal crystal system and develops mostly tetrahedral crystals as well as interpenetrating or cyclic twins , but also massive or grape-like mineral aggregates from gold to brass-like color. However, it leaves a greenish-black to black line color on the marking board . The crystals are opaque in any form and show a metallic sheen on the surface.
With a Mohs hardness of 3.5 to 4, chalcopyrite is one of the medium-hard minerals that, like the reference mineral fluorite, can be easily scratched with a pocket knife . However, the mineral reacts in a brittle manner to mechanical loads and breaks in a shell-like or uneven manner like glass .
Etymology and history
Chalcopyrite was first scientifically described by Johann Friedrich Henckel in 1725 and named after the Greek words chalkos for copper and pyros for fire.
An older term from Georgius Agricola (copper) gravel (also -kis , Latin: pyrites ) refers as a collective term to all hard sulfur, arsenic and antimony metal sulfides .
classification
Already in the outdated 8th edition of the mineral classification according to Strunz , chalcopyrite belonged to the mineral class of "sulfides and sulfosalts" and there to the department of "sulfides with the molar ratio metal (M): sulfur (S) = 1: 1", where it was classified as Namesake of the "chalcopyrite series" with the system no. II / B.02 with the other members Gallit , Raguinit , Roquesit and Talnakhit (formerly Roquésit).
In the Lapis mineral directory according to Stefan Weiß, which, out of consideration for private collectors and institutional collections, is still based on this classic system of Karl Hugo Strunz , the mineral was given the system and mineral number. II / C.03-10 . In the "Lapis system" this corresponds to the department "Sulphides with the molar ratio of metal: S, Se, Te ≈ 1: 1", where chalcopyrite is also named after the "chalcopyrite group" with the other members Eskebornit , Gallit, Laforêtit , Lenaite , Roquesite and Shenzhuangit forms (as of 2018).
The 9th edition of Strunz's mineral systematics , which has been in effect since 2001 and was updated by the International Mineralogical Association (IMA) until 2009, also classifies chalcopyrite in the category of “metal sulfides, M: S = 1: 1 (and similar)”. However, this is further subdivided according to the predominant metals in the compound, so that the mineral can be found in the sub-section "with zinc (Zn), iron (Fe), copper (Cu), silver (Ag) etc." according to its composition where, together with eskebornite, gallite, laforêtite, lenaite and roquesite, the "chalcopyrite group" with the system no. 2.CB.10a forms.
The systematics of minerals according to Dana , which is mainly used in the English-speaking world , assigns the to the class of "sulfides and sulfosalts" and there in the department of "sulfide minerals". Here it is together with eskebornite, gallite, roquesite, lenaite and laforêtite in the "chalcopyrite group (tetragonal: I 4 2 d )" with the system no. 02.09.01 within the sub-section " Sulphides - including selenides and tellurides - with the composition A m B n X p , with (m + n): p = 1: 1 ".
Chemism
Chalcopyrite has a theoretical composition of 34.6% copper, 30.5% iron and 34.9% sulfur and can usually be found in pure form in nature . However, it can contain traces of gold , silver and excess iron as an admixture (impurity) .
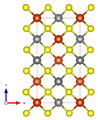
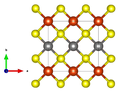
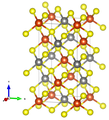
Crystal structure
Chalcopyrite crystallizes tetragonally in the space group I 4 2 d (space group no. 122) with the lattice parameters a = 5.29 Å and c = 10.42 Å and 4 formula units per unit cell .
In the crystal structure , the iron is tetrahedrally coordinated by four sulfur atoms in a perfect tetrahedral angle of 109.47 ° . The geometry of the copper is that of a flattened tetrahedron, the S-Cu-S angles being 108.68 ° and 111.06 °, respectively.
The X-ray crystallographic structural analyzes cannot conclusively provide any clear information about the oxidation states of iron or copper. It is assumed that the effective ion charge is between Cu + Fe 3+ (S 2− ) 2 and Cu 2+ Fe 2+ (S 2− ) 2 .
| Crystal structure of chalcopyrite |
|
|
| Color table: __ Cu __ Fe __ S |
properties
The mineral is sometimes confused with the cubic pyrite because of its golden sheen and its tetrahedral twin formation (interpenetrating twins of two sphenohedra; sphenoid = wedge-shaped crystal shape) . Chalcopyrite, however, has a more yellowish color and becomes colored over time due to weathering. Chalkopyrite is also confused with gold by laypeople .
When placed on carbon in front of the soldering tube , chalcopyrite melts easily into a gray-black, magnetic ball. It does not react to hydrochloric acid (HCl), but dissolves in nitric acid (HNO 3 ) with the separation of sulfur.
Chalcopyrite changes from 550 ° C into cubic crystallizing β-chalcopyrite.
Education and Locations


Chalcopyrite forms massive aggregates, but also often well-developed crystals in hydrothermal veins, various metamorphic and as so-called "run-through mineral" in all igneous rocks and igneous ores . It usually occurs in paragenesis with bornite and pyrite , with which it is occasionally confused due to the similar colors or temper colors, but also with many other copper or other metal sulfides such as sphalerite , galena and tetrahedrite as well as generally with barite , calcite , Dolomite and quartz .
The mineral turns into brown iron stone , brick ore , copper pechers and other copper salts such as malachite , azurite and chalcanthite through weathering .
Chalcopyrite has already been identified as a very common mineral formation at many sites around the world, with over 25,000 sites known to date (as of 2015).
The Nikolai mine near Dalnegorsk in Russia, where crystals up to 40 cm in size were discovered, is particularly well-known for its extraordinary chalcopyrite finds . Up to 12 cm large crystals and steps appeared in the Japanese mines Arakawa near Kyōwa (today Daisen ) and Osarizawa near Kazuno in the prefecture Akita on Honshū .
Other locations are in Afghanistan , Egypt , Albania , Argentina , Armenia , Azerbaijan , Australia , Belgium , Bolivia , Brazil , Bulgaria , Chile , China , Germany , Ecuador , Finland , France , Greece , Greenland , India , Indonesia , Iran , Ireland , Italy , Japan , Cambodia , Canada , Kazakhstan , Columbia , Democratic Republic of Congo , North and South Korea , Cuba , Madagascar , Morocco , Mexico , Myanmar , Namibia , New Zealand , Norway , Austria , Papua New Guinea , Peru , Philippines , Poland , Portugal , Romania , Russia , Zambia , Sweden , Switzerland , Zimbabwe , Slovakia , Spain , South Africa , Czech Republic , Turkey , Ukraine , Hungary , the United Kingdom (Great Britain), the United States of America (USA), Vietnam and Cyprus .
Chalcopyrite was also found in mineral samples from the Mid-Atlantic and Central Indian Ridge , the East Pacific Ridge and off Earth on the Moon .
use
raw material
Chalcopyrite is one of the most important copper ores; not so much because of its copper content (about 34% by weight), but because of its wide distribution.
Various substances from the group of chalcopyrites, which also includes chalcopyrite itself, can be used as active material in solar cells . So far (as of 2009) mixtures of chalcopyrites consisting of copper, indium, gallium, selenium and sulfur, Cu (In, Ga) (Se, S) 2 have dominated . These solar cells are often assigned to the general generic term CIGS solar cell, regardless of their exact composition .
Gemstone
Due to its low hardness, chalcopyrite is not suitable for commercial use as a gem stone , as it can be easily damaged (scratches, abrasion). For collectors, however, it is occasionally offered in the form of cabochon pendants or tumbled stones and hand flatterers .
Chalcopyrite can also be used to make handicrafts, similar to soapstone .
See also
literature
- Hans Jürgen Rösler : Textbook of Mineralogy . 4th revised and expanded edition. German publishing house for basic industry (VEB), Leipzig 1987, ISBN 3-342-00288-3 , p. 313-315 .
- Helmut Schrätze , Karl-Ludwig Weiner : Mineralogy. A textbook on a systematic basis . de Gruyter, Berlin; New York 1981, ISBN 3-11-006823-0 , pp. 155-164 .
- Friedrich Klockmann : Klockmann's textbook of mineralogy . Ed .: Paul Ramdohr , Hugo Strunz . 16th edition. Enke, Stuttgart 1978, ISBN 3-432-82986-8 , pp. 429-432 (first edition: 1891).
- Reiner Klenk, Thilo Glatzel, Alexander Grimm, C.-H. Fischer, Michael Kirsch, Iver Lauermann, Jörg Reichardt, Heike Steigert, Thomas P. Niesen, Sven Visbeck: The interface in chalcopyrite solar cells - a new approach . In: G. Stadermann (Ed.): Photovoltaics - new horizons . September 2003, p. 94–95 ( fvee.de [PDF; 324 kB ; accessed on October 9, 2019]).
Web links
- Mineral Atlas: Chalcopyrite (Wiki)
- Chalcopyrite search results. In: rruff.info. Database of Raman spectroscopy, X-ray diffraction and chemistry of minerals (RRUFF), accessed on October 11, 2019 .
- American-Mineralogist-Crystal-Structure-Database - Chalcopyrite. In: rruff.geo.arizona.edu. Retrieved October 11, 2019 .
- Chalcopyrite. In: mindat.org. Hudson Institute of Mineralogy, accessed October 11, 2019 .
Individual evidence
- ^ A b c Hugo Strunz , Ernest H. Nickel : Strunz Mineralogical Tables. Chemical-structural Mineral Classification System . 9th edition. E. Schweizerbart'sche Verlagbuchhandlung (Nägele and Obermiller), Stuttgart 2001, ISBN 3-510-65188-X , p. 77 (English).
- ↑ a b c Chalcopyrite . In: John W. Anthony, Richard A. Bideaux, Kenneth W. Bladh, Monte C. Nichols (Eds.): Handbook of Mineralogy, Mineralogical Society of America . 2001 (English, handbookofmineralogy.org [PDF; 65 kB ; accessed on October 11, 2019]).
- ^ German dictionary by Jacob Grimm and Wilhelm Grimm - Gelbkies. In: woerterbuchnetz.de. Berlin-Brandenburg Academy of Sciences, accessed on October 11, 2019 .
- ^ Helmut Schrätze , Karl-Ludwig Weiner : Mineralogie. A textbook on a systematic basis . de Gruyter, Berlin; New York 1981, ISBN 3-11-006823-0 , pp. 164 ( limited preview in Google Book search).
- ^ Georgius Agricola: De Natura Fossilium . Ed .: Fritz Krafft. Marix Verlag GmbH, Wiesbaden 2006, ISBN 3-86539-052-8 , p. 381 .
- ↑ Stefan Weiß: The large Lapis mineral directory. All minerals from A - Z and their properties. Status 03/2018 . 7th, completely revised and supplemented edition. Weise, Munich 2018, ISBN 978-3-921656-83-9 .
- ↑ Ernest H. Nickel, Monte C. Nichols: IMA / CNMNC List of Minerals 2009. (PDF 1703 kB) In: cnmnc.main.jp. IMA / CNMNC, January 2009, accessed October 8, 2019 .
- ↑ a b c Friedrich Klockmann : Klockmanns textbook of mineralogy . Ed .: Paul Ramdohr , Hugo Strunz . 16th edition. Enke, Stuttgart 1978, ISBN 3-432-82986-8 , pp. 430 (first edition: 1891).
- ↑ Hans Jürgen Rösler : Textbook of Mineralogy . 4th revised and expanded edition. German publishing house for basic industry (VEB), Leipzig 1987, ISBN 3-342-00288-3 , p. 313 .
- ^ Sydney R. Hall, James M. Stewart: The Crystal Structure Refinement of Chalcopyrite, CuFeS 2 . In: Acta Crystallographica . B29, 1973, p. 579 , doi : 10.1107 / S0567740873002943 (English).
- ^ Helmut Schrätze , Karl-Ludwig Weiner : Mineralogie. A textbook on a systematic basis . de Gruyter, Berlin; New York 1981, ISBN 3-11-006823-0 , pp. 159 ff .
- ↑ Localities for Chalcopyrite. In: mindat.org. Hudson Institute of Mineralogy, accessed October 11, 2019 .
- ↑ Records in the mineral sector. In: Mineralienatlas Lexikon. Stefan Schorn u. a., accessed on October 11, 2019 .
- Jump up ↑ Arakawa Mine, Kyohwa, Akita Prefecture, Tohoku Region, Honshu Island, Japan. In: mindat.org. Hudson Institute of Mineralogy, accessed October 11, 2019 .
- ↑ Petr Korbel, Milan Novák: Mineral Encyclopedia (= Dörfler Natur ). Edition Dörfler im Nebel-Verlag, Eggolsheim 2002, ISBN 978-3-89555-076-8 , p. 27 (as copper pebbles ) (Note: The "Arawaka" pit, which is given as a Japanese site, is probably a misspelling, see description of the site "Arakawa Mine" near Mindat).
- ↑ a b List of locations for chalcopyrite in the Mineralienatlas and Mindat , accessed on October 11, 2019
- ^ David Barthelmy: Chalcopyrite Mineral Data. In: webmineral.com. Retrieved October 11, 2019 .
- ↑ Carsten Deibel, Vladimir Dyakonov: Chalcopyrite-based thin-film solar cells. University of Oldenburg , archived from the original on February 12, 2013 ; accessed on October 11, 2019 .
- ^ Walter Schumann: Precious stones and gemstones. All kinds and varieties. 1900 unique pieces . 16th, revised edition. BLV Verlag, Munich 2014, ISBN 978-3-8354-1171-5 , pp. 222 .