Cobalt
| properties | |||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General | |||||||||||||||||||||||||||||||||||||||||||||||||
| Name , symbol , atomic number | Cobalt, Co, Jan. | ||||||||||||||||||||||||||||||||||||||||||||||||
| Element category | Transition metals | ||||||||||||||||||||||||||||||||||||||||||||||||
| Group , period , block | 9 , 4 , d | ||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | metallic with a bluish-greyish hue | ||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number | 7440-48-4 | ||||||||||||||||||||||||||||||||||||||||||||||||
| EC number | 231-158-0 | ||||||||||||||||||||||||||||||||||||||||||||||||
| ECHA InfoCard | 100.028.325 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Mass fraction of the earth's envelope | 37 ppm | ||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic | |||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic mass | 58,933194 (4) et al | ||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius (calculated) | 135 (152) pm | ||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | low-spin: 126 pm, high-spin: 150 pm | ||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [ Ar ] 3 d 7 4 s 2 | ||||||||||||||||||||||||||||||||||||||||||||||||
| 1. Ionization energy | 7th.88101 (12) eV ≈ 760.4 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| 2. Ionization energy | 17th.0844 (12) eV ≈ 1 648.39 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| 3. Ionization energy | 33.50 (6) eV ≈ 3 232.3 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| 4. Ionization energy | 51.27 (10) eV ≈ 4 947 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| 5. Ionization energy | 79.50 (20) eV ≈ 7 671 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| Physically | |||||||||||||||||||||||||||||||||||||||||||||||||
| Physical state | firmly | ||||||||||||||||||||||||||||||||||||||||||||||||
| Modifications | 2 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | hexagonal | ||||||||||||||||||||||||||||||||||||||||||||||||
| density | 8.90 g / cm³ (20 ° C ) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 5.0 | ||||||||||||||||||||||||||||||||||||||||||||||||
| magnetism | ferromagnetic | ||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 1768 K (1495 ° C) | ||||||||||||||||||||||||||||||||||||||||||||||||
| boiling point | 3173 K (2900 ° C) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Molar volume | 6.67 · 10 −6 m 3 · mol −1 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of evaporation | 390 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 17.2 kJ mol −1 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound | 4720 m · s −1 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Specific heat capacity | 421 J kg −1 K −1 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Work function | 5.0 eV | ||||||||||||||||||||||||||||||||||||||||||||||||
| Electric conductivity | 16.7 · 10 6 A · V −1 · m −1 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 100 W m −1 K −1 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Chemically | |||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 2 , 3 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Normal potential | −0.28 V (Co 2+ + 2 e - → Co) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 1.88 ( Pauling scale ) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
| For other isotopes see list of isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||
| NMR properties | |||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
| safety instructions | |||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . |
|||||||||||||||||||||||||||||||||||||||||||||||||
Cobalt (chemical terminology; latin Cobaltum , standard language cobalt ; from Erstbeschreiber after the cobalt ore as a starting material Cobalt Rex named) is a chemical element with the element symbol Co and the atomic number 27, Cobalt is a ferromagnetic transition metal of Group 9 or cobalt group of the Periodic Table . In the older counting method it belongs to the 8th subgroup or iron-platinum group .
history
Cobalt ores and cobalt compounds have been known for a long time and were primarily used as cobalt blue ( Thénards Blau and Zaffer ) for coloring glass and ceramics . In the Middle Ages they were often thought of as valuable silver and copper ores . However, since they could not be processed and because of the arsenic content gave off bad smells when heated, they were viewed as bewitched. Allegedly goblins ate the precious silver and excreted worthless silver-colored ores in its place . In addition to cobalt, these were also tungsten and nickel ores. These ores were then nicknamed by the miners with nicknames such as nickel, tungsten (for example "Wolfs-Schaum", lat. Lupi spuma ) and Kobold ore, i.e. cobalt. In 1735, the Swedish chemist Georg Brandt discovered the previously unknown metal while processing cobalt ores, described its properties and gave it its current name. In 1780, Torbern Olof Bergman discovered that cobalt is an element while studying its properties more closely.
properties
Physical Properties
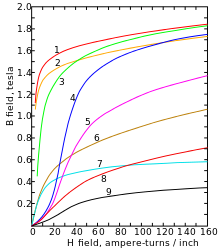
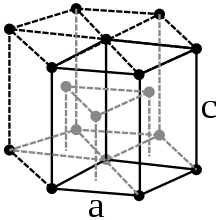
Cobalt is a steel-gray, very tough heavy metal with a density of 8.89 g / cm³. It is ferromagnetic with a Curie temperature of 1150 ° C. Cobalt occurs in two modifications : a hexagonal densest ( hcp ) crystal structure in the space group P 6 3 / mmc (space group no.194 ) with the lattice parameters a = 250.7 pm and c = 406.9 pm as well as two formula units per Unit cell and a face-centered cubic shape ( fcc ) with the lattice parameter a = 354.4 pm. The hcp modification (α-cobalt, historically ε-cobalt) is stable at lower temperatures and converts to the fcc modification (β-cobalt, historically α-cobalt) at approx. 450 ° C.
As a typical metal , it conducts heat and electricity well; its electrical conductivity is 26 percent of that of copper .
The atomic mass of naturally occurring cobalt is a special feature; At 58.93 it is greater than the mean atomic mass of nickel at 58.69, the next element in the periodic table . This peculiarity also exists between argon and potassium and between tellurium and iodine .
Chemical properties
Its chemical behavior is similar to that of iron and nickel , and it is resistant in air through passivation ; it is only by oxidizing acting acids dissolved. With a normal potential of −0.277 V, cobalt is one of the base elements . In compounds it occurs mainly in the oxidation states + II and + III. However, the oxidation states −I, 0, + I, + II, + III, + IV and + V are also represented in compounds. Cobalt forms a multitude of mostly colored complexes . In contrast to covalent compounds , the oxidation state + III is more common and more stable than + II.
Isotopes

A total of 30 isotopes and 18 further core isomers between 47 Co and 77 Co are known. Natural cobalt consists entirely of the isotope 59 Co. The element is therefore one of the 22 pure elements . This isotope can be examined by means of NMR spectroscopy .
The nuclide 57 Co decays to 57 Fe via electron capture . The gamma radiation emitted during the transition to the basic state of the daughter nucleus has an energy of 122.06 keV (85.6%) and 14.4 keV (9.16%). The main application of 57 Co is Mössbauer spectroscopy to differentiate between divalent and trivalent iron.
The most durable of the unstable isotopes is 60 Co ( cobalt-60 , spin 5 + ) connected to a half-life of 5.27 years under beta decay initially in an excited state of 60 Ni (Spin 4 + ) and subsequently with emission of gamma radiation (two Gamma quanta of energy 1.17 and 1.33 MeV ) in the ground state (spin 0 + ) of this nuclide decays. For this reason, 60 Co as a source of gamma radiation for sterilization or preservation of food, the material examination ( radiographic testing ) and in cancer therapy ( " cobalt therapy used"). Other isotopes such as 57 Co or 58 Co can also be used as tracers in medicine. 60 Co is exclusively obtained artificially from 59 Co through neutron activation . Spontaneous fission sources such as 252 Cf serve as the neutron source for the production of smaller quantities , while 59 Co-pellets are exposed to the neutron flux in nuclear reactors to produce larger quantities .
The formation of 60 Co from 59 Co under neutron radiation could potentially also be used to intensify the effect of nuclear weapons, in which neutron radiation is generated by being coated with cobalt ( cobalt bomb ). In the event of a detonation, the strong gamma emitter would then be formed, which would contaminate the environment more strongly than the core explosion alone. If 60 Co is not properly disposed of but melted down with other cobalt and processed into steel , steel parts made from it can be radioactive to a harmful extent.
The Wu experiment was carried out with 60 Co , through which the parity violation of the weak interaction was discovered.
Occurrence
Cobalt is a rare element with an abundance in the earth's crust of 0.004 percent. This puts it in thirtieth position in the list of items sorted by frequency. Elementary it occurs only extremely rarely in meteorites and in the earth's core . Cobalt is found in many minerals , but mostly only occurs in small amounts. The element is always associated with nickel, often also with copper, silver, iron or uranium . Nickel is about three to four times as common as cobalt. Both metals belong to the siderophilic elements and are characteristic of basic and ultra-basic magmatites .
Cobalt is found as a trace element in most soils. There are a number of cobalt ores in which the cobalt has accumulated through weathering or other processes. The most important are: cobaltite (obsolete cobalt gloss ; CoAsS) linnaeite and Siegenit (obsolete and misleading cobalt nickel gravel ; (Co, Ni) 3 S 4 ), Erythrin (obsolete cobalt bloom ), Asbolan (obsolete Erdkobalt ) Skutterudit ( smaltite , Smaltin , CoAs 3 ) and heterogenite (CoOOH). The cobalt content of the sulfidic ores is low, mostly only 0.1–0.3 percent.
The world's known cobalt reserves are 25 million tons . The most important ore deposits are in the Democratic Republic of the Congo and in Zambia , where cobalt occurs together with copper , as well as in Canada , Morocco , Cuba , Russia , Australia and the USA . Another 120 million tons of cobalt are believed to be in the earth's crust on the floors of the Atlantic , Pacific and Indian Oceans . The cobalt occurs there in manganese nodules together with manganese , copper and nickel and can be obtained as a metal alloy in a smelting reduction furnace.
Due to the importance of cobalt in the manufacture of electronic devices, there could be a shortage of supply in the coming years.
Extraction and presentation
Cobalt is mainly extracted from copper and nickel ores . The exact method of extraction depends on the composition of the original ore. First of all, some of the iron sulfides ( FeS and FeS 2 ) present are converted into iron oxide by roasting and then slagged with silicon dioxide as iron silicate . The so-called raw stone is created , which in addition to cobalt also contains nickel, copper and other iron as sulfide or arsenide . Further sulfur is removed by roasting with sodium carbonate and sodium nitrate . Sulphates and arsenates are formed from part of the sulfur and arsenic , which are leached with water. The corresponding metal oxides remain and are treated with sulfuric or hydrochloric acid . Only copper does not dissolve, while nickel, cobalt and iron dissolve. With chlorinated lime , cobalt can then be selectively precipitated as cobalt hydroxide and thus separated. This is converted into cobalt (II, III) oxide (Co 3 O 4 ) by heating and then reduced to cobalt with coke or aluminum powder :
The majority of cobalt is obtained by reducing the cobalt by-products of nickel and copper mining and the smelting . Because cobalt is typically a by-product, the supply of cobalt is highly dependent on the economics of copper and nickel mining in a particular market.
There are several methods of separating cobalt from copper and nickel, depending on the concentration of cobalt and the exact composition of the ore used . One method is foam flotation, in which surfactants bind to various ore constituents, which leads to an enrichment of cobalt ores. Subsequent roasting converts the ores into cobalt (II) sulfate and oxidizes copper and iron . By washing out with water, the sulfate is extracted together with the arsenates . The residues are further leached with sulfuric acid , resulting in a copper sulfate solution . Cobalt can also be leached from the copper melt.
advancement
States with the largest production volume
| country | 2006 | 2013 | 2016 |
|---|---|---|---|
| DR Congo | 22000 | 54000 | 66000 |
| China | 1400 | 7200 | 7700 |
| Canada | 5600 | 6920 | 7300 |
| Russia | 5100 | 6300 | 6200 |
| Brazil | 1000 | 3000 | 5800 |
| Australia | 6000 | 6400 | 5100 |
| Zambia | 8600 | 5200 | 4600 |
| Cuba | 4000 | 4200 | 4200 |
| Philippines | 3000 | 3500 | |
| New Caledonia | 1100 | 3190 | 3300 |
| South Africa | 3000 | 3000 | |
| Morocco | 1500 | 1700 | |
| remaining countries | 1200 | 8000 | 8300 |
| total | 57,500 | 110,000 | 123,000 |
Cobalt producers
In many cases, cobalt is not refined in the countries in which cobalt ores are mined. The following table from the Cobalt Development Institute (CDI) lists the producers of metallic cobalt and cobalt salts and their production quantities in tons:
| Surname | country | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CDI co- members |
Ambatovy | Madagascar | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2083 | 2915 | 3464 | 3273 |
| BHPB / QNPL | Australia | 1800 | 1900 | 1400 | 1600 | 1800 | 1600 | 1700 | 2141 | 2631 | 2369 | |||||
| CTT | Morocco | 1431 | 1593 | 1613 | 1405 | 1591 | 1711 | 1600 | 1545 | 1788 | 1314 | 1353 | 1391 | 1722 | 1568 | |
| Eramet | France | 181 | 199 | 280 | 256 | 305 | 311 | 368 | 302 | 354 | 326 | 308 | 219 | 133 | 119 | |
| Freeport Cobalt, formerly OMG | Finland | 7990 | 7893 | 8170 | 8580 | 9100 | 8950 | 8850 | 9299 | 10441 | 10547 | 10010 | 11452 | 8582 | 11187 | |
| Gécamines | DR Congo | 1200 | 735 | 600 | 550 | 606 | 300 | 415 | 745 | 650 | 870 | 700 | 500 | 400 | 400 | |
| Glencore , Katanga | DR Congo | 2800 | 2900 | 0 | ||||||||||||
| Glencore, Minara | Australia | 2900 | 3300 | 3200 | ||||||||||||
| Glencore, Mopani Copper | Zambia | 0 | 0 | 0 | ||||||||||||
| Glencore, formerly Xstrata | Norway | 4556 | 4670 | 5021 | 4927 | 3939 | 3719 | 3510 | 3208 | 3067 | 2969 | 3400 | 3600 | 3100 | 3500 | |
| ICCI | Canada | 3141 | 3225 | 3391 | 3312 | 3573 | 3428 | 3721 | 3706 | 3853 | 3792 | 3319 | 3210 | 3733 | 3693 | |
| Rubamine | India | 0 | 517 | 579 | 200 | 45 | ||||||||||
| Sumitomo | Japan | 379 | 429 | 471 | 920 | 1084 | 1071 | 1332 | 1935 | 2007 | 2542 | 2747 | 3654 | 4259 | 4305 | |
| Umicore | Belgium | 1704 | 2947 | 3298 | 2840 | 2825 | 3020 | 2150 | 2600 | 3187 | 4200 | 5415 | 5850 | 6306 | 6329 | |
| Vale Inco | Canada | 1000 | 1562 | 1563 | 1711 | 2033 | 2200 | 1193 | 940 | 2070 | 1890 | 2240 | 2051 | 1858 | 1851 | |
| Chambishi Metals | Zambia | 4570 | 3769 | 3648 | 3227 | 2635 | 2591 | 235 | 3934 | 4856 | 5435 | 5000 | 4317 | 2997 | 4725 | |
| Total CDI | 27,952 | 28,922 | 29,455 | 29,328 | 29,491 | 28,901 | 25,074 | 30,872 | 35,483 | 36,454 | 36,620 | 44,859 | 42,754 | 44,150 | ||
| Non- CDI co- members |
China | 4576 | 8000 | 12700 | 12700 | 13245 | 18239 | 2544 | 35929 | 34969 | 29784 | 36062 | 39292 | 48719 | 45046 | |
| India | 255 | 545 | 1220 | 1184 | 980 | 858 | 1001 | 670 | 720 | 600 | 250 | 100 | 150 | 100 | ||
| Glencore, Katanga | DR Congo | 0 | 0 | 0 | 0 | 0 | 749 | 2535 | 3437 | 2433 | 2129 | 2300 | ||||
| Kasese | Uganda | 0 | 457 | 638 | 674 | 698 | 663 | 673 | 624 | 661 | 556 | 376 | 0 | 0 | 0 | |
| Glencore, Minara | Australia | 2039 | 1979 | 1750 | 2096 | 1884 | 2018 | 2350 | 1976 | 2091 | 2400 | 2700 | ||||
| Glencore, Mopani Copper | Zambia | 2050 | 2022 | 1774 | 1438 | 1700 | 1450 | 1300 | 1092 | 1100 | 230 | 0 | ||||
| Norilsk Nickel | Russia | 4654 | 4524 | 4748 | 4759 | 3587 | 2502 | 2352 | 2460 | 2337 | 2186 | 2368 | 2302 | 2040 | 3092 | |
| QNPL | Australia | 2281 | 2519 | 1850 | 0 | |||||||||||
| South Africa | 285 | 300 | 214 | 257 | 307 | 244 | 236 | 833 | 840 | 1100 | 1294 | 1332 | 1300 | 1101 | ||
| Votorantim | Brazil | 1097 | 1155 | 1136 | 902 | 1148 | 994 | 1012 | 1369 | 1613 | 1750 | 1653 | 1350 | 1300 | 400 | |
| DLA | United States | 1987 | 1632 | 1199 | 294 | 617 | 203 | 180 | −8 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total non-CDI | 16,943 | 20,614 | 25,379 | 24,304 | 24,166 | 27,920 | 37.183 | 48,382 | 46764 | 40,735 | 49.284 | 46,895 | 55,359 | 49,739 | ||
| total | 44,895 | 49,536 | 54,834 | 53,632 | 53,657 | 56,821 | 59,851 | 79,252 | 82,247 | 77.189 | 85.904 | 91,754 | 98.113 | 93,889 | ||
Notes A:
- ↑ a b not a CDI member since 2013
- ↑ a b c estimated
- ↑ a b c d CDI member since 2014
- ↑ CDI member from 2009 to 2013
- ↑ including Umicore China
- ↑ without Umicore China
- ↑ including Nicomet and Rubamin (not 2009 to 2013)
- ↑ not a CDI member since 2009
- ↑ Defense Logistic Agency : Sale of cobalt from US strategic reserves
- ↑ does not include quantities from manufacturers who do not publish their production
use

Cobalt was first used in the form of oxides , sulfates , hydroxides or carbonates for heat-resistant paints and pigments , e.g. B. used for painting porcelain and ceramics (see also smalts and blue color works ). This was followed by the most famous decorative application in the form of blue cobalt glass . After 1800 the cobalt aluminate (CoAl 2 O 4 ) was produced industrially as a strong color pigment.
Today cobalt is used as an alloy component to increase the heat resistance of alloyed and high-alloy steels , especially high-speed steel and superalloys , as a binder phase in hard metals and diamond tools (see: Widia ). Its use as an alloying element and in cobalt compounds makes it a strategically important metal. (See Vitallium : implants , turbine blades , chemical apparatus.) Cobalt steels are used e.g. B. for highly stressed work parts that have to withstand high temperatures, such. B. valve seat rings in internal combustion engines or guide vanes in gas turbines .
Cobalt- based superalloys have historically consumed most of the cobalt produced. The temperature stability of these alloys makes them suitable for turbine blades for gas turbines and aircraft engines , although single crystal nickel-based alloys outperform. Cobalt-based alloys are also resistant to corrosion and wear , so like titanium they can be used to make orthopedic implants that will not wear out over time. The development of wear-resistant cobalt alloys began in the first decade of the 20th century with the stellite alloys , which contained chromium with different proportions of tungsten and carbon . Alloys with chromium and tungsten carbides are very hard and wear-resistant. Special cobalt-chrome- molybdenum alloys such as Vitallium are used for prosthetic parts. Cobalt alloys are also used in dentures as a useful substitute for nickel , which may be allergenic . Some high speed steels also contain cobalt to increase heat resistance and wear resistance. The special alloys of aluminum , nickel, cobalt and iron , known as alnico , as well as samarium and cobalt (samarium-cobalt magnet ) are used in permanent magnets .
Cobalt is a component of magnetic alloys, as a dryer ( siccative ) for paints and lacquers , as a catalyst for desulphurization and hydrogenation , as hydroxide or lithium cobalt dioxide (LiCoO 2 ) in batteries , in corrosion and wear-resistant alloys and as a trace element for Medicine and agriculture . Cobalt is also used in the production of magnetic data carriers such as audio tapes and video cassettes , where it is doped to improve the magnetic properties. Cobalt has been used as an alloy component for guitar strings for some time .

Since lithium-ion accumulators came onto the market in the 1990s, cobalt has been used for accumulators , especially for mobile applications, since the lithium-cobalt oxide accumulator has a particularly high energy density. The first commercially available lithium-ion battery came onto the market as a lithium-cobalt dioxide battery from Sony in 1991. Due to the expected increasing importance of accumulators for mobile electronics and electromobility , the Federation of German Industries (BDI) advises : “Due to the high concentration of cobalt deposits in the politically unstable states of the Congo and Zambia, research into manganese and iron phosphate electrodes and Also recommended in principle in nickel electrodes that contain little or no cobalt ". In a position paper of the BDI on innovative drive technologies it is predicted: "The global raw material demand for cobalt could increase by 3.4 times by the year 2030 compared to 2006 by the increasing demand for lithium-ion batteries alone".
Lithium cobalt (III) oxide is widely used in lithium ion battery cathodes . The material consists of cobalt oxide layers with embedded lithium . During the discharge, the lithium is released as lithium ions. Nickel-cadmium batteries and nickel-metal hydride batteries also contain cobalt to improve the oxidation of nickel in the battery.
Although most of the cobalt was used in batteries in a mobile device in 2018 , rechargeable batteries for electric cars are a more recent application for cobalt. This industry has quintupled its demand for cobalt, making it imperative to find new raw materials in more stable areas of the world. Even if the cobalt content of new batteries is declining, demand is expected to increase over the next few years as the spread of electric vehicles increases. In 2017, electric mobility accounted for 8.2% of global cobalt consumption; including all other areas of application, battery production was responsible for around 46% of cobalt consumption. As of 2019, modern batteries contain only around a third of the cobalt of older batteries due to better cell designs; the car manufacturer Tesla is already working on largely cobalt-free batteries. LiFePo batteries, such as those used for solar batteries , on the other hand, do not require any cobalt at all.
Catalysts based on cobalt are in reactions with carbon monoxide used. Cobalt is also a catalyst in the Fischer-Tropsch process for the hydrogenation of carbon monoxide to liquid fuels . In the hydroformylation of alkenes is often cobalt octacarbonyl as a catalyst, although it is often more efficient catalysts iridium - and rhodium is replaced, for. B. the Cativa process .
In the hydrodesulfurization of petroleum , a catalyst is used that is derived from cobalt and molybdenum . This process helps purify the petroleum of sulfur impurities that affect the refining of liquid fuels.
physiology
Cobalt is part of vitamin B 12 , cobalamin , which is essential for human survival . In healthy people, this vitamin can possibly be formed directly from cobalt ions by intestinal bacteria . However, cobalamin must be bound by the intrinsic factor produced in the stomach in order to be able to be absorbed in the ileum . However, since the production site of man-made cobalamin is in the large intestine , absorption is not possible according to the current state of knowledge. The vitamin must therefore be taken in through food. Nevertheless, a daily intake of 0.1 μg cobalt is specified as a trace element for the daily requirement for adults. The lack of vitamin B 12 can lead to impaired erythropoiesis and thus to anemia . In ruminants , such a deficiency is mainly due to insufficient cobalt intake. In animal production , traces of cobalt are added to the feed if the animals have to be fed on pastures that are poor in cobalt . This is intended to counteract growth and lactation disorders , anemia and loss of appetite .
Bacteria in the stomach of ruminants convert cobalt salts into vitamin B 12 , a compound that can only be produced by bacteria or archaea . A minimal presence of cobalt in soils therefore significantly improves the health of grazing animals.
On cobalamin -based proteins use Corrin to keep the cobalt. Coenzyme B 12 has a reactive C-Co bond that is involved in the reactions . In humans, B 12 has two types of alkyl ligands: methyl and adenosyl . Methylcobalamin promotes the transfer of methyl groups . The adenosyl version of B 12 catalyzes rearrangements in which a hydrogen atom is transferred directly between two adjacent atoms with simultaneous exchange of the second substituent X, which can be a carbon atom with substituents, an oxygen atom of an alcohol or an amine . Methylmalonyl-CoA mutase converts methylmalonyl-CoA into succinyl-CoA , an important step in the production of energy from proteins and fats .
Although much rarer than other metalloproteins (e.g. zinc and iron ), other cobaltoproteins are known besides B 12 . These proteins include methionine aminopeptidase 2, an enzyme found in humans and other mammals that does not use the corrin ring of B 12 but instead binds cobalt directly. Another uncorrinated cobalt enzyme is nitrile hydratase, an enzyme in bacteria that metabolizes nitriles .
While small overdoses of cobalt compounds are only slightly toxic to humans, larger doses of 25 to 30 mg per day lead to skin, lung and stomach diseases, liver, heart, kidney damage and cancerous ulcers.
A number of cases of cobalt-induced cardiomyopathy (cobalt cardiomyopathy) occurred in Canada and the United States in the mid-1960s . 49 patients were registered in Quebec and 64 in Omaha. Symptoms included stomach pain, weight loss, nausea, shortness of breath, and coughing, among others. The mortality rate was 40 percent. Autopsies revealed severe damage to the heart muscle and liver . All patients were heavy beer drinkers, consuming 1.5 to 3 liters per day. They preferred to consume varieties from local breweries that had started adding cobalt (II) sulfate to beer as a foam stabilizer about a month earlier . The limit values for cobalt in food were not exceeded. The incidence of the disease came to a standstill immediately after the breweries discontinued the cobalt (II) sulfate admixtures.
Cobalt (II) salts activate the hypoxia -inducible transcription factors ( HIF ) and increase the expression of HIF -dependent genes . This includes the gene for erythropoietin (EPO). Cobalt (II) salts could be abused by athletes to promote the formation of red blood cells .
proof

A relatively informative preliminary sample for cobalt is the phosphorus salt pearl , which is colored intensely blue by cobalt ions. In the cation separation process it can be detected in addition to nickel with thiocyanate and amyl alcohol ; when dissolved in amyl alcohol it forms blue Co (SCN) 2 . The red-violet cobalt (II) thiocyanate in water also turns blue when mixed with acetone .
Cobalt can be quantitatively determined with EDTA in a complexometric titration against murexide as an indicator .
links
Cobalt is usually bivalent or trivalent in its compounds . These compounds often have strong colors. Important cobalt compounds are:
Oxides
Cobalt (II) oxide is an olive-green salt that is insoluble in water . It forms a sodium chloride structure of space group Fm 3 m (space group no. 225) . Cobalt (II) oxide is used as a raw material for the production of pigments , in particular for the production of the pigment smalt , which is also used in the ceramic industry . It can also be used to make cobalt glass as well as Thénards blue . Cobalt (II, III) oxide is a black solid and belongs to the group of spinels .
Cobalt (II, III) oxide is an important intermediate product in the production of metallic cobalt. By roasting and leaching , cobalt (II, III) oxide is first obtained from various cobalt ores (mostly sulfides or arsenides ). This can now be reduced to the element with carbon or aluminothermically .
Cobalt (III) oxide is a gray-black solid that is practically insoluble in water. At a temperature above 895 ° C, it splits off oxygen , forming cobalt oxides such as Co 3 O 4 and CoO.
Halides

Cobalt (II) chloride is a blue salt when anhydrous and pink when it is hexahydrate . It has a trigonal crystal structure of the cadmium (II) hydroxide type with the space group P 3 m 1 (space group no. 164) . Anhydrous cobalt (II) chloride is very hygroscopic and easily absorbs water. It changes its color very characteristically from blue to pink. The opposite color change from pink to blue is also possible by heating the hexahydrate to temperatures above 35 ° C. Because of the typical color change , it was used as a moisture indicator in desiccants such as silica gel . With the help of cobalt (II) chloride, water can also be detected in other solutions . It is also used as a so-called secret ink , as it is hardly visible as a hexahydrate in aqueous solution on the paper , but when it is heated, deep blue writing emerges.
Cobalt (II) bromide is a green, hygroscopic solid that changes to the red hexahydrate in air . It is easily soluble in water with a red color. Cobalt (II) iodide is a black, graphite-like hygroscopic mass that slowly turns black-green in air. It is soluble in water, whereby the diluted solutions look red, concentrated solutions turn red at low temperatures and all shades from brown to olive green at higher temperatures. Cobalt (II) bromide and cobalt (II) iodide have a hexagonal cadmium iodide - crystal structure of the space group P 6 3 mc (. Space group No. 186) .
More connections
Cobalt (II) nitrate is a salt of nitric acid , formed from the cobalt cation and the nitrate anion . The brown-red and hygroscopic salt is usually in the form of a hexahydrate and forms monoclinic crystals that are readily soluble in water, ethanol and other organic solvents .
Cobalt (II) oxalate is a flammable, hardly inflammable, crystalline, pink solid that is practically insoluble in water. It decomposes when heated above 300 ° C. It occurs in two allotropic crystal structures. One has a monoclinic crystal structure with the space group C 2 / c (space group no. 15) , the other an orthorhombic crystal structure with the space group Cccm (space group no. 66) . Cobalt (II) oxalate is mainly used to make cobalt powder. The yellowish-pink tetrahydrate is used in the manufacture of catalysts .
Cobalt (II) sulfate is a violet-tinged red, hygroscopic salt in the anhydrous state . Cobalt (II) sulphate is used for the production of pigments , glazes , in porcelain painting , for toning paper ( photography ), in baths for cobalt electroplating and for trace element supplementation in aquaristics, among others.
Cobalt yellow is a fine, light crystalline powder and is used as a pigment for oil and watercolor painting .
Thénards Blue is a blue pigment made by sintering cobalt (II) oxide with aluminum oxide at 1200 ° C. It is extremely stable and has been used in the past as a dye for ceramics (especially Chinese porcelain), jewelry, and paints . Transparent glasses are tinted with the cobalt pigment smalt based on silicon dioxide .
The pigment Rinmans Green is a turquoise green powder and is mainly used for oil paints and cement paints . Rinman's green is a popular proof of zinc . Zinc oxide or zinc hydroxide is mixed with a small amount of a highly diluted cobalt nitrate solution on a magnesia channel . Rinman's green is created when glowing weakly in the oxidizing flame .
Cobalt complexes

When ammonia solution is added, a cobalt (II) chloride solution initially forms a precipitate of cobalt (II) hydroxide , which dissolves in an excess of ammonia solution and ammonium chloride in the presence of atmospheric oxygen as an oxidizing agent to form various ammine-cobalt (III) complexes . The orange-yellow hexaammine cobalt (III) chloride and the red aquapentaammine cobalt (III) chloride are formed in particular .
In addition, various chloroammine cobalt (III) complexes can also be formed, such as chloropentaammine cobalt (III) chloride or dichlorotetraammine cobalt (III) chloride. Some of these compounds fall out of solution . In addition, there are also ammine complexes of cobalt (II) salts, such as hexaammine cobalt (II) sulphate, which can be produced by passing ammonia gas over anhydrous cobalt (II) sulphate .
In addition to the ammine complexes, there are numerous compounds with different ligands . Examples are potassium hexacyanocobaltate (II) (K 4 [Co (CN) 6 ]), potassium tetrathiocyanatocobaltate (II) (K 2 [Co (SCN) 4 ]), potassium hexanitritocobaltate (III) (Fischer's salt, cobalt yellow ), and complexes with organic ligands such as ethylenediamine or the oxalate ion .
One property of [Co (NH 3 ) 5 (NO 2 )] Cl (NO 3 ) is remarkable . When exposed to UV light, the micrometer to millimeter-sized crystals jump in this cobalt coordination compound and cover distances that are a thousand times their size. This is due to isomerization of the nitrite ligand (NO 2 ), which leads to tension in the crystal. This conversion of light into mechanical energy has been studied by scientists from the United Arab Emirates and Russia.
See also
literature
- AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , pp. 1681–1695.
- Michael Binnewies: General and Inorganic Chemistry. 1st edition, Spektrum Verlag , Heidelberg 2004, ISBN 3-8274-0208-5 .
- Harry H. Binder: Lexicon of the chemical elements - the periodic table in facts, figures and data. S. Hirzel Verlag , Stuttgart 1999, ISBN 3-7776-0736-3 .
Web links
- Mineral Atlas: Cobalt (Wiki)
Individual evidence
- ↑ a b c Harry H. Binder: Lexicon of chemical elements , S. Hirzel Verlag, Stuttgart 1999, ISBN 3-7776-0736-3 .
- ↑ The values for the properties (info box) are taken from www.webelements.com (Cobalt) , unless otherwise stated .
- ↑ CIAAW, Standard Atomic Weights Revised 2013
- ↑ a b c d e entry on cobalt in Kramida, A., Ralchenko, Yu., Reader, J. and NIST ASD Team (2019): NIST Atomic Spectra Database (ver. 5.7.1) . Ed .: NIST , Gaithersburg, MD. doi : 10.18434 / T4W30F ( https://physics.nist.gov/asd ). Retrieved June 11, 2020.
- ↑ a b c d e entry on cobalt at WebElements, https://www.webelements.com , accessed on June 11, 2020.
- ↑ a b N. N. Greenwood and A. Earnshaw: Chemistry of the elements , 1st edition, VCH, Weinheim 1988, ISBN 3-527-26169-9 , p. 1427.
- ↑ a b Yiming Zhang, Julian RG Evans, Shoufeng Yang: Corrected Values for Boiling Points and Enthalpies of Vaporization of Elements in Handbooks. In: Journal of Chemical & Engineering Data. 56, 2011, pp. 328-337, doi: 10.1021 / je1011086 .
- ^ John Dallas Donaldson, Detmar Beyersmann: Cobalt and Cobalt Compounds. In: Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag, Weinheim 2005, doi: 10.1002 / 14356007.a07_281.pub2 (access via subscribed institutions).
- ↑ Ludwig Bergmann, Clemens Schaefer, Rainer Kassing: Textbook of Experimental Physics , Volume 6: Solid . 2nd edition, Walter de Gruyter, 2005, ISBN 978-3-11-017485-4 , p. 361.
- ↑ a b Entry on cobalt in the GESTIS substance database of the IFA , accessed on August 9, 2016 (JavaScript required)
- ↑ Entry on cobalt in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Claus Priesner, Karin Figala: Alchemy: Lexicon of a Hermetic Science . CH Beck, January 1, 1998, ISBN 978-3-406-44106-6 , p. 199.
- ↑ a b c d Center D'Information du Cobalt (Ed.): Cobalt Monograph . Prepared in collaboration with the staff of Battelle Memorial Institute, Columbus, Ohio. M. Weissenbruch, Brussels 1960, OCLC 921191777 (English, 515 pages).
- ↑ Joachim Heimannsberg: Brockhaus! What is not in the dictionary. ISBN 3-7653-1551-6 , pp. 255-256.
- ^ Charles Steinmetz: Theory and Calculation of Electric Circuits . Editor: McGraw-Hill. 1917, Fig. 42 .
- ↑ a b K. Schubert: A model for the crystal structures of the chemical elements . In: Acta Crystallographica . 1974, B30, pp. 193-204, doi: 10.1107 / S0567740874002469 .
- ^ A b A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1682.
- ^ B. Lee, R. Alsenz, A. Ignatiev, M. Van Hove, M. Hove: Surface structures of the two allotropic phases of cobalt . In: Physical Review B . tape 17 , no. 4 , 1978, p. 1510–1520 , doi : 10.1103 / PhysRevB.17.1510 .
- ^ A b Hans Breuer: dtv-Atlas Chemie. Vol. 1, 9th edition, dtv Verlagsgesellschaft , Munich 2000, ISBN 3-423-03217-0 .
- ↑ G. Audi, FG Kondev, Meng Wang, WJ Huang, S. Naimi: The NUBASE2016 evaluation of nuclear properties. In: Chinese Physics C. 41, 2017, S. 030001, doi: 10.1088 / 1674-1137 / 41/3/030001 ( full text ).
- ↑ Table of Isotopes decay data.
- ^ Georgia State University: Cobalt-60 at HyperPhysics.
- ↑ Centers for Disease Control and Prevention (CDC): Cobalt-60. ( Memento of the original from November 30, 2005 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. Atlanta 2004, accessed February 21, 2009.
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1681.
- ^ Cobalt at rutherford-online - Lexicon of the Elements 2006 .
- ^ Christian Schwägerl : Radiation scrap was distributed all over Germany. In: Spiegel Online from February 17, 2009.
- ^ Oak Ridge Associated Universities: Contaminated Pipe Fitting from Taiwan.
- ↑ Chien-Shiung Wu : Experimental Test of Parity Conservation in Beta Decay . In: Physical Reviews 105, 1957, pp. 1413-1415 ( doi: 10.1103 / PhysRev.105.1413 )
- ↑ Mineralienatlas: cobalt nickel gravel (Wiki).
- ↑ a b c Cobalt 2014 at USGS Mineral Resources (PDF file; 28 kB).
- ↑ Marcus Sommerfeld, David Friedmann, Thomas Kuhn, Bernd Friedrich: “Zero-Waste”: A Sustainable Approach on Pyrometallurgical Processing of Manganese Nodule Slags . In: Minerals . 8, No. 12, 23 November 2018, p. 544. doi : 10.3390 / min8120544 .
- ↑ Akram Belkaïd: Cobalt is getting scarce. Retrieved July 10, 2020 .
- ↑ Kim B. Shedd: Mineral Yearbook 2006: Cobalt . United States Geological Survey. Retrieved October 26, 2008.
- ↑ Kim B. Shedd: Commodity Report 2008: Cobalt . United States Geological Survey. Retrieved October 26, 2008.
- ^ Henry Sanderson: Cobalt's meteoric rise at risk from Congo's Katanga , Financial Times. March 14, 2017.
- ^ Davis, Joseph R .: ASM specialty handbook: nickel, cobalt, and their alloys . ASM International, 2000, ISBN 0-87170-685-7 , p. 347.
- ↑ Cobalt 2007 at USGS Mineral Resources (PDF file; 60 kB).
- ↑ Cobalt 2016 at USGS Mineral Resources (PDF file; 28 kB).
- ^ Official website of the Cobalt Development Institute
- ↑ Cobalt Development Institute: Cobalt_news_april12 (PDF; 2 MB)
- ↑ Cobalt Development Institute: 14-2_cobalt_news (PDF; 1.6 MB)
- ↑ Cobalt Development Institute: 17-2_cobalt_news (PDF; 1.6 MB)
- ↑ Sebastian Weber, Frederic van gen Hassend: Iron instead of cobalt - A way to sustainable use of raw materials . University of Wuppertal . Retrieved June 11, 2019.
- ↑ Kim B. Shedd: Mineral Yearbook 2006: Cobalt . United States Geological Survey. Retrieved October 26, 2008.
- ↑ Kim B. Shedd: Commodity Report 2008: Cobalt . United States Geological Survey. Retrieved October 26, 2008.
- ^ Matthew J. Donachie: Superalloys: A Technical Guide . ASM International, 2002, ISBN 978-0-87170-749-9 .
- ^ Flake C. Campbell: Cobalt and Cobalt Alloys . In: Elements of metallurgy and engineering alloys June 30, 2008, ISBN 978-0-87170-867-0 , pp. 557-558.
- ^ R. Michel: Systemic effects of implanted prostheses made of cobalt-chromium alloys . In: Archives of Orthopedic and Trauma Surgery . 110, No. 2, 1991, pp. 61-74. doi : 10.1007 / BF00393876 . PMID 2015136 .
- ↑ John A. Disegi: Cobalt-base Aloys for Biomedical Applications . ASTM International, 1999, ISBN 0-8031-2608-5 , p. 34.
- ^ FE Luborsky: Reproducing the Properties of Alnico Permanent Magnet Alloys with Elongated Single-Domain Cobalt-Iron Particles . In: Journal of Applied Physics . 28, No. 344, 1957. bibcode : 1957JAP .... 28..344L . doi : 10.1063 / 1.1722744 .
- ^ M. Hawkins: Why we need cobalt . In: Applied Earth Science: Transactions of the Institution of Mining and Metallurgy, Section B . 110, No. 2, 2001, pp. 66-71. doi : 10.1179 / aes.2001.110.2.66 .
- ^ RD Armstrong, GWD Briggs, EA Charles: Some effects of the addition of cobalt to the nickel hydroxide electrode . In: Journal of Applied Electrochemistry . 18, No. 2, 1988, pp. 215-219. doi : 10.1007 / BF01009266 .
- ↑ P. Zhang, Toshiro Yokoyama, Osamu Itabashi, Yoshito Wakui, Toshishige M. Suzuki, Katsutoshi Inoue: Recovery of metal values from spent nickel – metal hydride rechargeable batteries . In: Journal of Power Sources . 77, No. 2, 1999, pp. 116-122. bibcode : 1999JPS .... 77..116Z . doi : 10.1016 / S0378-7753 (98) 00182-7 .
- ↑ Castellano, Robert (2017-10-13) How To Minimize Tesla's Cobalt Supply Chain Risk . Seeking Alpha .
- ↑ As Cobalt Supply Tightens, LiCo Energy Metals Announces Two New Cobalt Mines . CleanTechnica, November 28, 2017. Accessed January 7, 2018.
- ↑ #Faktenfuchs: Cobalt demand is increasing because of e-mobility . In: Bayerischer Rundfunk , November 17, 2019. Retrieved July 13, 2020.
- ↑ Martin Doppelbauer: Strategy paper electric cars - current status and future development (V1.5) . Karlsruhe Institute of Technology . Retrieved November 7, 2019.
- ↑ Khodakov, Andrei Y .: Advances in the Development of Novel Cobalt Fischer-Tropsch Catalysts for Synthesis of Long-Chain Hydrocarbons and Clean Fuels . In: Chemical Reviews . 107, No. 5, 2007, pp. 1692-1744. doi : 10.1021 / cr050972v . PMID 17488058 .
- ^ Hebrard, Frédéric: Cobalt-Catalyzed Hydroformylation of Alkenes: Generation and Recycling of the Carbonyl Species, and Catalytic Cycle . In: Chemical Reviews . 109, No. 9, 2009, pp. 4272-4282. doi : 10.1021 / cr8002533 . PMID 19572688 .
- ^ M. Hawkins: Why we need cobalt . In: Applied Earth Science: Transactions of the Institution of Mining and Metallurgy, Section B . 110, No. 2, 2001, pp. 66-71. doi : 10.1179 / aes.2001.110.2.66 .
- ↑ Schmidt, Lang: Physiologie des Menschen. 30th edition, p. 856.
- ↑ Kurt Hausmann: The importance of intestinal bacteria for the vitamin B 12 and folic acid supply of humans and animals. In: Klinische Wochenschrift , edition 33/1955, number 15-16, pp. 354-359, doi: 10.1007 / BF01467965 .
- ↑ Cem Ekmekcioglu, Wolfgang Marktl: Cobalt deficiency. In: Essential trace elements: Clinic and nutritional medicine , Springer 2006, ISBN 978-3-211-20859-5 , p. 198 ( limited preview in the Google book search).
- ↑ Wolfgang Löscher, Fritz Rupert Ungemach, Reinhard Kroker: Vitamin B12. In: Pharmacotherapy for domestic and farm animals , 7th edition, Georg Thieme Verlag 2006; P. 346. ISBN 978-3-8304-4160-1 .
- ↑ Hans-Konrad Biesalski, Stephan C. Bischoff, Christoph Puchstein (Eds.): 11.4 Cobalt. In: Nutritional medicine: according to the new nutritional medicine curriculum of the German Medical Association , 4th edition, Georg Thieme Verlag 2010; ISBN 978-3-13-100294-5 , p. 205.
- ^ FJ Schwarz, M. Kirchgessner, GI Stangl: Cobalt requirement of beef cattle - feed intake and growth at different levels of cobalt supply . In: Journal of Animal Physiology and Animal Nutrition . 83, No. 3, 2000, pp. 121-131. doi : 10.1046 / j.1439-0396.2000.00258.x .
- ^ Judith G. Voet, Donald Voet: Biochemistry . J. Wiley & Sons, New York 1995, ISBN 0-471-58651-X , p. 675, OCLC 31819701 .
- ↑ David M. Smith, Bernard T. Golding, Leo Radom: Understanding the Mechanism of B12-Dependent Methylmalonyl-CoA Mutase: Partial Proton Transfer in Action . In: Journal of the American Chemical Society . 121, No. 40, 1999, pp. 9388-9399. doi : 10.1021 / ja991649a .
- ↑ Michihiko Kobayashi: Cobalt proteins . In: European Journal of Biochemistry . 261, No. 1, 1999, pp. 1-9. doi : 10.1046 / j.1432-1327.1999.00186.x . PMID 10103026 .
- ↑ C. Thomas: Special Pathology. Schattauer Verlag, 1996, ISBN 3-7945-2110-2 , p. 179 ( limited preview in the Google book search).
- ↑ Expert Group on Vitamins and Minerals. 2002.
- ^ Cardiology: When Beer Brought the Blues. In: The New York Times , January 10, 1967 issue.
- ↑ Wolfgang Jelkmann: The Disparate Roles of Cobalt in Erythropoiesis, and Doping Relevance . In: Open Journal of Hematology . tape 3 , 2012, p. 3–6 , doi : 10.13055 / ojhmt_3_1_6.121211 .
- ↑ a b Heinrich Remy : Textbook of Inorganic Chemistry. Vol. II, Academic Publishing Company Geest & Portig Leipzig 1961, pp. 356–365.
- ^ E. Merck : Complexometric determinations with Titriplex. Darmstadt.
- ^ A b Jean D'Ans, Ellen Lax: Paperback for chemists and physicists. 3. Elements, inorganic compounds and materials, minerals, Volume 3. 4. Edition, Springer, 1997, ISBN 978-3-540-60035-0 , p. 386 ( limited preview in the Google book search).
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1685.
- ↑ Cobalt dibromide. In: webelements.com. Retrieved June 21, 2017 (English).
- ↑ D. Nicholls: The Chemistry of Iron, Cobalt and Nickel: Comprehensive Inorganic Chemistry . Elsevier, 2013, ISBN 978-1-4831-4643-0 , pp. 1070 ( limited preview in Google Book Search).
- ^ E. Romero, ME Mendoza, R. Escudero: Weak ferromagnetism in cobalt oxalate crystals. In: physica status solidi. 248, 2011, p. 1519, doi: 10.1002 / pssb.201046402 .
- ^ Ullmann's Encyclopedia of Industrial Chemistry . John Wiley & Sons, 2003, ISBN 3-527-30385-5 , pp. 785 ( limited preview in Google Book search).
- ↑ D. Nicholls: The Chemistry of Iron, Cobalt and Nickel Comprehensive Inorganic Chemistry . Elsevier, 2013, ISBN 978-1-4831-4643-0 , pp. 1072 ( limited preview in Google Book search).
- ↑ C. Thomas: Special Pathology. Schattauer Verlag, 1996, ISBN 3-7945-2110-2 , p. 179 ( limited preview in the Google book search).
- ↑ AF Gehlen: About the preparation of a blue color from cobalt, which is just as beautiful as ultramarine. Archived from the original on February 10, 2018 by the citizen Thenard . In: H. Frölich. (Ed.): New general journal of chemistry, Volume 2 . 1803.
- ↑ Jander-Blasius: '' Introduction to the inorganic-chemical internship ''. 14th edition 1995.
- ↑ Panče Naumov, Subash Chandra Sahoo et al. a .: Dynamic Single Crystals: Kinematic Analysis of Photoinduced Crystal Jumping (The Photosalient Effect). In: Angewandte Chemie. 125, 2013, pp. 10174-10179, doi: 10.1002 / anie.201303757 .










![{\ displaystyle \ mathrm {2 \ CoCl_ {2} +2 \ NH_ {4} Cl + 10 \ NH_ {3} +1/2 \ O_ {2} \ longrightarrow 2 \ [Co (NH_ {3}) _ { 6}] Cl_ {3} + H_ {2} O}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/3bb13aee50b91f22617e0736f7f43095f3ff87cb)
![{\ displaystyle \ mathrm {2 \ CoCl_ {2} +2 \ NH_ {4} Cl + 8 \ NH_ {3} +1/2 \ O_ {2} \ longrightarrow 2 \ [Co (H_ {2} O) ( NH_ {3}) _ {5}] Cl_ {3}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/462adb052c102f0fd32915f78463b3ab8c0b6687)
![{\ displaystyle \ mathrm {CoSO_ {4} +6 \ NH_ {3} \ longrightarrow [Co (NH_ {3}) _ {6}] SO_ {4}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/c97ce856150ed5f62d7f11315245510a7d32a645)