Chemical reaction
A chemical reaction is a process in which one or more chemical compounds are converted into others and energy is released or absorbed. Also, elements may be involved in reactions. Chemical reactions are usually associated with changes in the chemical bonds in molecules or crystals . Due to a chemical reaction, the properties of the products can change significantly compared to the starting materials . The chemical reactions do not include physical processes in which only the physical state changes, such as melting or evaporation , diffusion , the mixing of pure substances into mixtures of substances, and nuclear reactions in which elements are converted into others.
Reactions usually consist of a rather complicated sequence of individual sub-steps, the so-called elementary reactions , which together form the overall reaction. The reaction mechanism provides information on the exact sequence of the partial steps . For the description of chemical reactions which is reaction equation used are shown graphically in the starting materials, products, and sometimes important intermediates and via an arrow, the reaction arrow to be connected to each other.
Both elementary reactions and reaction mechanisms can be divided into different groups. Elementary reactions include, for example, the disintegration of one molecule into two or the reverse, the synthesis of two atoms or molecules into one. Reaction mechanisms are often classified according to the change in the substances involved. If there is a change in the oxidation numbers , one speaks of oxidation and reduction ; a solid product is created from dissolved substances, from a precipitation .
The extent to which a certain reaction between two or more partners takes place depends on the difference in the Gibbs energy of the products and the educts, which is composed of an enthalpic and an entropic component . With negative values, the reaction equilibrium is on the side of the products. However, there are also many reactions that are thermodynamically possible in this sense , but proceed kinetically very slowly, in extreme cases so slow that they can practically not be observed. The reason for this is an activation energy that is too high , which must be applied so that the further reaction is possible. Such reactions take place faster at higher temperatures, since a comparatively larger number of the particles involved have enough energy to overcome the activation barrier. In many reactions this is also possible by means of catalysis , in which not the direct reaction takes place, but a different one in which a third substance, which is unchanged from the reaction, is involved. The presence of this catalyst reduces the activation energy required.
history
Chemical reactions such as burning in fire , alcoholic fermentation or the reduction of ores to metals - for example in the case of iron - have been known for a very long time. The first theories for the transformation of substances were developed by Greek philosophers, such as the four-element theory of Empedocles , according to which every substance is composed of the four basic elements fire, water, air and earth and can also be broken down into these. In the Middle Ages, the alchemists were mainly concerned with chemical reactions. In particular, they tried to convert lead into gold , using, among other things, reactions of lead and lead-copper alloys with sulfur .
The production of chemical substances that do not occur in nature through suitable reactions has long been known. This concerns, for example, sulfuric and nitric acid , the first production of which is attributed to the controversial alchemist Jabir ibn Hayyan . It was made by heating sulphate and nitrate ores such as vitriol, alum and saltpeter. In the 17th century, Johann Rudolph Glauber produced hydrochloric acid and sodium sulfate for the first time by reacting sulfuric acid and sodium chloride . With the development of the lead chamber process for sulfuric acid production and the Leblanc process for sodium carbonate production, chemical reactions were also used industrially. With increasing industrialization, industrial synthesis became more important and newer and more efficient methods were developed. Examples are the contact process for sulfuric acid production, which was used from 1870, or the Haber-Bosch process for ammonia synthesis developed in 1910 .
From the 16th century onwards, researchers such as Johan Baptista van Helmont , Robert Boyle or Isaac Newton tried to scientifically investigate observed chemical transformations and set up theories about their process. An important reaction studied was combustion , for which Johann Joachim Becher and Georg Ernst Stahl developed the phlogiston theory in the early 18th century . However, this turned out to be wrong and could be refuted in 1785 by Antoine Lavoisier , who found the correct explanation of the combustion as a reaction with oxygen in the air.
In 1808 Joseph Louis Gay-Lussac recognized that gases always react with one another in certain proportions. From this and from Dalton's atomic theory, Joseph Louis Proust developed the law of constant proportions , on which stoichiometry is based and which also enabled the development of the reaction equations.
For a long time it was assumed that organic reactions are determined by a special " life force " (vis vitalis) and thus differ from non-organic reactions. After Friedrich Wöhler synthesized urea from inorganic precursors in 1828, this assumption lost much of its importance in chemistry. Other chemists who made important contributions to the elucidation of organic chemical reactions were, for example, Justus von Liebig with his radical theory , Alexander William Williamson , who developed the synthesis of ethers named after him , and Christopher Kelk Ingold , who, among other things, researched the mechanisms for substitution reactions .
Reaction equations
So-called reaction equations are used to graphically represent chemical reactions . These consist of sum or structural formulas of the starting materials on the left and those of the products on the right. In between there is an arrow, the so-called reaction arrow, which shows the direction and type of reaction. The tip of the arrow always points in the direction in which the reaction is taking place. In equilibrium reactions , double arrows are used that point in opposite directions. Reaction equations should be stoichiometrically balanced. This means that there should be the same number of atoms on both sides of the reaction arrow and that equations should be balanced by different numbers of molecules involved.
- Schematic simple reaction equation
More complicated reactions are represented by formula schemes which, in addition to starting materials and products, also show important intermediate products or transition states. The reaction paths are illustrated by arrows, which show the attack of electron pairs from one atom on other atoms. In reaction equations in organic chemistry, small, inorganic molecules such as water or carbon dioxide are often placed on the arrow (for educts) or below (for products) or indicated by a sign. Also, catalysts , solvents , special conditions or other substances that play a role during the reaction, however, do not change with this are written on the reaction arrow.
 Typical reaction mechanism in organic chemistry (example: Baeyer-Villiger oxidation based on the reaction of a percarboxylic acid with a ketone )
Typical reaction mechanism in organic chemistry (example: Baeyer-Villiger oxidation based on the reaction of a percarboxylic acid with a ketone )
The notation of a reaction as a retrosynthesis can also be useful when planning complicated syntheses . Here a reaction is written down from the end, i.e. the product, which is broken down through possible synthesis steps until possible starting materials are reached. Retrosyntheses are indicated by a special arrow, the retrosynthesis arrow ( ).
Elementary reactions
The elementary reaction is the smallest section into which a chemical reaction can be broken down. Macroscopically observable reactions are built up from a large number of elementary reactions that take place in parallel or one after the other. The specific sequence of individual elementary reactions is also known as the reaction mechanism . One or two, rarely three molecules are usually involved in an elementary reaction. Reactions with more molecules are practically impossible as it is extremely unlikely that more than three molecules will get close enough to react at the same time.
The most important elementary reactions are the unimolecular and the bimolecular reactions. In a unimolecular reaction, only one molecule is involved, which is converted into one or more other molecules through isomerization or disintegration. These reactions usually require an input of energy in the form of heat or irradiation with light.
An example of a typical unimolecular reaction is the cis - trans isomerization , in which the cis form of a compound is converted to the trans form or vice versa.

When dissociating , a bond in a molecule splits and two parts are created. The cleavage can take place homo- or heterolytically . In the first case, the bond is split in such a way that each part retains an electron and radicals are formed; in the case of heterolytic cleavage, both electrons stay with one part of the molecule, while the other does not retain any electrons from the split bond and so ions are formed. Decays play an important role in triggering chain reactions such as the oxyhydrogen reaction or polymerizations .
- Disintegration of a molecule AB into two smaller parts A and B.
In bimolecular reactions, two molecules collide and react with each other. One possibility is that these two molecules become one, i.e. a synthesis takes place. This happens, for example, when two radicals react to form a molecule. Even with addition reactions in organic chemistry, a new one is formed from several molecules.
However, it is also possible that a reaction does not produce a stable molecule and only part of one molecule is transferred to the other. This type of reaction occurs, for example, in redox and acid-base reactions. In redox reactions, the transferred particle is an electron, in acid-base reactions it is a proton. This type of reaction is also called metathesis .
Chemical equilibrium
Every chemical reaction in a homogeneous phase is reversible and can proceed in both directions. If, for example, two substances react to form a third, the third also breaks down into the starting substances. There and back reactions are always in competition with each other and differ in terms of different reaction speeds . Since reaction rates are also concentration-dependent, they change over time. The speeds of the back and forth reaction converge more and more as the reaction progresses until they are finally the same. At this point in time, the concentrations of the individual substances in the reaction mixture no longer change, an equilibrium, the so-called chemical equilibrium, is reached.
The position of the equilibrium depends not only on the properties of the substances involved, but also on the temperature and pressure and is determined by the minimum free energy. Often the derivation of the free enthalpy, the free enthalpy of reaction, is calculated, which must be 0 in equilibrium. The pressure dependency can easily be explained with the principle of Le Chatelier , according to which a system evades a constraint such as a pressure increase in such a way that the effect is minimal.
At this point, the maximum yield of a reaction has been reached, since with further formation of a product the reverse reaction now proceeds faster and is therefore preferred until equilibrium is reached again. However, higher yields can be achieved by removing products from the reaction mixture in which the equilibrium is disturbed, or by changing the pressure or temperature. The initial concentrations of the substances involved have no influence on the position of the equilibrium.
thermodynamics
Chemical reactions are largely determined by the laws of thermodynamics. In principle, every reaction takes place. However, in very many cases the equilibrium is almost entirely on the side of the educts. In order for a reaction to take place, it must be exergonic , i.e. the free enthalpy must decrease during the reaction. The free enthalpy is made up of two different thermodynamic quantities, the enthalpy and the entropy . These are linked to one another via the fundamental equation for the free enthalpy.
- G: free enthalpy, H: enthalpy, T: temperature, S: entropy, Δ: differences
Reactions can take place in several ways. One possibility is the exothermic reaction , in which Δ H is negative and energy is released. Depending on the amount of energy released, highly ordered structures with low entropy can also arise. Typical examples of exothermic reactions with loss of entropy are precipitations and crystallizations , in which ordered solid structures arise from disordered structures in the gas phase, liquid or solution. In the case of endothermic reactions, however, heat is consumed and has to be absorbed from the environment. These can only take place if the entropy of the system increases at the same time. This can take place, for example, via the formation of gaseous reaction products, which have a high entropy.
Since the entropy is temperature-dependent and increases with increasing temperature, entropy-specific reactions such as decays preferably take place at high temperatures. Energy-specific reactions such as crystallization, on the other hand, take place primarily at low temperatures. Sometimes the direction of a reaction can be reversed by changing the temperature.
An example of this is the Boudouard equilibrium .
The reaction of carbon dioxide and carbon to form carbon monoxide is endothermic, so that the equilibrium at low temperatures is on the side of the carbon dioxide. Only at temperatures of over 800 ° C is this side preferred due to the higher entropy on the side of the carbon monoxide.
Reactions can also be viewed through changes in internal energy . This can also be described using a fundamental equation that takes into account entropy, volume changes and chemical potential , among other things . The latter depends, among other things, on the activities of the substances involved.
- U: internal energy, S: entropy, p: pressure, μ: chemical potential, n: amount of substance, d: differential notation
Reaction kinetics
Reaction kinetics investigate the rate at which a reaction occurs. This depends on various parameters of the reaction, such as the order of the reaction , the concentrations of the substances involved, the temperature , the activation energy and other, mostly empirically determined, factors. There are also various theories to theoretically calculate reaction rates for different systems at the molecular level. In contrast to reaction kinetics, this field of work is also referred to as reaction dynamics.
For elementary reactions, simple rate laws can be drawn up, which differ depending on the order of the reaction and show the dependence on the concentrations of the substances involved. For a reaction of the first order, i.e. a decay of a substance A, the following applies to the reaction rate v ( k : rate constant, t : time, [A]: concentration of A, [A] 0 : initial concentration of A):
integrated
In a first-order reaction, the rate of reaction depends only on the concentration and the properties of the decomposing substance. Since the concentration decreases exponentially with time in a 1st order reaction , a constant half-life that is typical for the respective reaction can be determined. This value is often given, especially in the case of radioactive decays that do not belong to the chemical reactions but also follow a first-order rate law . For other reaction orders and more complicated reactions there are correspondingly different rate laws. To calculate the rate constant, the Arrhenius equation can be used, which shows the temperature dependence of the constant.
A simple model with which the molecular course of a chemical reaction and the reaction rates can be explained is the collision theory . However, only a few simple reactions can be calculated reasonably correctly with this. For more complicated reactions, more precise theories, which are usually tailored to a specific problem, must be used. These include the theory of the transition state , the calculation of the potential energy surface , the Marcus theory and the RRKM theory .
Types of reactions
Reactions can be divided into different types, which differ in the type of particle transferred and the products formed.
Oxidation and reduction
If electrons are transferred during a reaction between two atoms , the oxidation states of the atoms involved change . The atom that gives off one or more electrons ( called reducing agent ) is oxidized, the other, the oxidizing agent , is reduced accordingly. Since both reactions always occur together, one also speaks of a redox reaction.
Which of the reactants involved is the reducing or oxidizing agent can be predicted on the basis of the electronegativities of the elements involved. Elements with low electronegativities, like most metals , easily donate electrons and are accordingly oxidized, whereas non-metals with high electronegativities are slightly reduced. If ions are involved in a redox reaction, the oxidation level of the ion must also be taken into account. Thus, chromates or permanganates in which the elements in high oxidation states are present, strong oxidizing agents.
How many electrons an element releases or absorbs in a redox reaction can often be predicted by the electron configuration of the reactants. Elements try to achieve the noble gas configuration and therefore often release or accept a corresponding number of electrons. This applies in particular to many main group elements such as the alkali metals , alkaline earth metals or halogens . For transition metals and especially heavy atoms, however, this often does not apply due to the high charge required to achieve the noble gas configuration and the increasing influence of relativistic effects . The noble gases , which already have a noble gas configuration, accordingly have no tendency to absorb further electrons and are very inert.
An important class of redox reactions are the electrochemical reactions. In electrolysis , the electrons in the electric current act as a reducing agent. Electrochemical reactions take place in galvanic cells , in which reduction and oxidation take place spatially separated. These reactions are particularly important for the production of many elements such as chlorine or aluminum . The reverse reaction, in which electrons are released in redox reactions and can be used as electrical energy, is also possible. This is the principle of the battery, in which energy is chemically stored and converted into electrical energy.
Complex formation reaction
In the complex formation reaction, several ligands react with a metal atom to form a complex . This occurs because lone pairs of electrons on the ligands penetrate into empty orbitals of the metal atom and form a coordinative bond . The ligands are Lewis bases that have lone pairs of electrons. This can be both ions and neutral molecules (such as carbon monoxide, ammonia or water). The number of ligands that react with the central metal atom can often be predicted with the help of the 18-electron rule , which can be used to determine particularly stable complexes. According to the crystal field and ligand field theory , the geometry of the complex also plays an important role; tetrahedral or octahedral complexes are particularly common .
Reactions can also take place within a complex. These include ligand exchange , in which one or more ligands are replaced by another, rearrangements and redox processes in which the oxidation level of the central metal atom changes.
Acid-base reactions
Acid-base reactions are - in the Brønsted definition of acids - reactions in which protons are transferred from one molecule to another. The proton is always transferred from the acid (proton donor) to the base (proton acceptor) (protolysis).
- Acid-base reaction, HA: acid, B: base, A - : corresponding base, HB + : corresponding acid
Since the transfer of the proton from the acid to the base produces a base and an acid, the so-called corresponding acids or bases, the reverse reaction is also possible. The acid / base and the corresponding base / acid are therefore always in equilibrium. Which side of the reaction the equilibrium lies on can be determined by the acid constants of the substances involved. The stronger an acid or base, the easier it is to release or absorb the proton. A special case of the acid-base reaction is neutralization , in which an acid and a base react in exactly the same ratio that a neutral solution, i.e. a solution without an excess of hydroxide or oxonium ions, is formed.
precipitation
Precipitation is a reaction in which previously dissolved particles combine and become a new, water-insoluble substance, the precipitate. This occurs mainly with dissolved ions, which come together when the solubility product is exceeded and form an insoluble salt . This can be done, for example, by adding a precipitant with a low solubility product to an already dissolved salt or by removing the solvent. Depending on the conditions, a substance can turn out very differently from a solution. If the precipitation takes place quickly, the ions do not have time to organize themselves, and an amorphous or microcrystalline precipitate forms. If the solubility product is slowly exceeded and there is supersaturation, on the other hand, precipitation takes place only slowly. The ions therefore have time to organize themselves and regular crystals are formed . This can also be done by recrystallization from the microcrystalline precipitate.
Solid state reactions
Reactions can also take place between two solid substances. However, the diffusion , which largely determines the speed of the reaction, is very small, so solid-state reactions are correspondingly slow reactions. This means that solid-state reactions generally have to be carried out at high temperatures. At the same time, the reactants should be distributed as finely as possible, since this creates the largest possible surface on which the two substances can react.
Photochemical reactions
Electromagnetic radiation plays a crucial role in photochemical reactions . Light and UV radiation with a wavelength of around 200 to 800 nm are of particular importance . This radiation excites electrons in atoms and molecules, and excited states are formed . These are very energetic due to the absorbed photons and can release the energy through various processes. In addition to physical processes such as fluorescence and phosphorescence , reactions are also possible here. Often homolytic bond breaks occur, so that radicals arise. For example, chain reactions such as the oxyhydrogen reaction of hydrogen and oxygen can be triggered by photochemical reactions . But ionizations , electron transfer reactions , isomerizations or rearrangements can also be caused by photochemical reactions.
A very biologically important photochemical reaction is photosynthesis , in which organic compounds from carbon dioxide and water are synthesized with the help of light . Photochemical reactions also play an important role in atmospheric chemistry , for example in the build-up and breakdown of ozone .
catalysis
In catalysis, the reaction of two substances does not take place directly, but via a detour. There is always a third substance involved, the so-called catalyst , which intervenes in the reaction, but in the end always emerges unchanged from the reaction. Through catalysis, reactions that are kinetically inhibited by a high activation energy can take place while this activation energy is bypassed. As a result, only a small amount of energy is used, and a reaction can thus be carried out economically. Sometimes reactions are only made possible by catalysts if, for example, competitive reactions take place preferentially at otherwise necessary temperatures.
Catalysts can be present both in a different phase (heterogeneous) and in the same phase (homogeneous). Heterogeneous catalysts are mostly solid bodies, on the surface of which the reactions take place. Accordingly, the surface of the catalyst should be as large as possible for effective catalysis. Catalytic reactions on surfaces are often associated with chemisorption , in which a molecule is chemically bound to the surface and therefore the bonds within the molecule are weakened. This enables an easier reaction.
Of particular importance in heterogeneous catalysis are the platinum metals and other transition metals, which are used in many technically important reactions such as hydrogenation , catalytic reforming or the synthesis of basic chemicals such as nitric acid or ammonia . Catalysts of homogeneous catalysis can be acids that increase the nucleophilicity of a carbonyl group and thus enable a reaction with otherwise non-reacting electrophiles, or soluble complexes as in hydroformylation .
Homogeneous catalysts have the advantage that there are no problems with the accessibility of the catalyst and that the surface area is too small; the reactants and the catalyst can easily be brought together by mixing and stirring. In addition, the catalyst, such as a complex, can be specifically and reproducibly synthesized for a reaction. A disadvantage, however, is the difficulty in separating the catalyst from the product, which can lead to contamination and loss of the usually expensive catalyst. This is why heterogeneous catalysts are preferred in many industrial processes.
Reactions in Organic Chemistry
In organic chemistry, in addition to the reactions that also take place with inorganic substances, such as oxidations, reductions or acid-base reactions, there are a number of other reactions in which covalent bonds are formed between carbon atoms or carbon and heteroatoms (e.g. oxygen, nitrogen, halogens) . In addition to the distinction between homolytic and radical reactions, these are classified primarily according to the type of structural change. Many special reactions in organic chemistry are named as name reactions after their discoverers.
substitution
In substitution, one atom, part of a molecule or ligand (in complex chemistry, where substitutions are also possible) is exchanged for another. An attacking atom or molecule takes the place of another atom or molecule split off as a leaving group . The bonding of the carbon atom does not change.
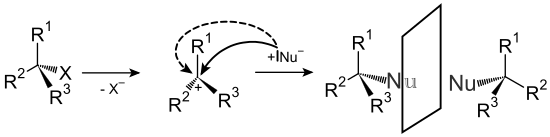
Substitution reactions can be divided into three types depending on the attacking particle. In the case of nucleophilic substitutions , a nucleophile , i.e. an atom or molecule with an excess of electrons and thus a negative charge or partial charge , attacks a suitable carbon atom and replaces another atom or part of the molecule. Typical nucleophiles are atoms, ions or groups of atoms with electronegative non-metals such as amines , halides , thiols , hydroxides or alcoholates . In addition to the nucleophile, the leaving group also plays a role in determining whether a substitution takes place. Good leaving groups should be easy to split off and form molecules or ions that are as stable as possible. Examples are the heavy halides bromide and iodide or nitrogen. This type of reaction is mainly found with aliphatic hydrocarbons , with aromatics - since aromatics have a high electron density - it is rather rare and can only take place under special circumstances with very strongly electron-withdrawing groups on the aromatics ( nucleophilic aromatic substitution ). Nucleophilic substitutions can take place by two different mechanisms, referred to as S N 1 and S N 2. The names are derived from the order of reactions according to which the rate-determining steps of the two types of reaction take place.
In the S N 1 mechanism, the leaving group is first split off, creating a carbocation . A rapid reaction with the nucleophile then takes place.
In the S N 2 mechanism, the nucleophile first attacks, forming a common transition state, and only then is the leaving group split off. The two mechanisms differ in the stereochemistry of the products obtained, with S N 1 a racemization occurs due to the trivalent carbocation , while with S N 2 a reversal of the previously existing stereochemistry ( Walden reversal ) is observed.
The counterpart to nucleophilic substitution is electrophilic substitution . In this case, an electrophile, i.e. an atom or molecule with a lower electron density, i.e. a positive charge or partial charge, is the attacking particle. Typical electrophiles are, for example, carbocations, the carbon atom in carbonyl groups , sulfur trioxide or nitronium cations. This reaction takes place almost exclusively with aromatic hydrocarbons, which is why one often speaks of an electrophilic aromatic substitution . In the mechanism, the attack of the electrophile initially forms the so-called σ complex, a transition state in which the aromatic system is suspended. The leaving group, usually a proton, is then split off and the aromatic system restored.
In the third type of substitution, the attacking particle is a radical , which is why it is also referred to as a radical substitution . This takes place in the form of a chain reaction and takes place, for example, in the reaction of alkanes with halogens. In the first step, for example, a few starter radicals are formed by light, heat or the decay of very unstable molecules. In the chain reaction, the reaction continues through the transfer of the radical until the chain breaks off through the recombination of two radicals.
- Reactions during the chain reaction of a radical substitution
Addition / elimination
The addition and the counterpart, the elimination , are reactions in which the number of substituents on the carbon atom changes and multiple bonds are formed or cleaved. In elimination reactions, double and triple bonds are created by removing (“eliminating”) a substituent on each carbon atom of the bond. For an elimination, there must be a suitable leaving group on a carbon atom of the bond in question, which can be split off relatively easily. Similar to nucleophilic substitution, there are several possible mechanisms that take place depending on the molecule and conditions and are in turn named according to the respective reaction order. In the E1 mechanism, the leaving group is initially split off with the formation of a carbocation. In the next step, the double bond is formed with splitting off of a proton. Due to the similar conditions of the two reactions, the E1 elimination is always in competition with the S N 1 substitution.
The first reaction order is also the E1cb mechanism, in which the proton is split off with the help of a base and a carbanion is formed. In the next step, the double bond is formed with the leaving group being split off.
The E2 mechanism also requires a base. In this case, however, the attack of the base and the elimination of the leaving group take place in concert and no ionic intermediate is formed. In contrast to the E1 eliminations, the stereochemistry in the product can be determined here, since a reaction of the base in the anti- position to the leaving group takes place preferentially. Due to similar conditions and reagents, the E2 elimination is always in competition with the S N 2 substitution.
The counterpart to elimination is the addition reaction . In this, atoms or molecules attach to double or triple bonds and form single bonds. Addition reactions can take place both on CC multiple bonds, i.e. alkenes or alkynes , and on carbon-heteroatom multiple bonds, such as carbonyl groups , thiocarbonyl groups or nitriles . Like the substitutions, the additions can also be divided into several groups depending on the attacking particle. In electrophilic addition , an electrophile, often a proton, attacks the double bond to form a carbenium ion. This reacts with nucleophiles to form the product.
There are two possibilities for the formation of the carbenium ion - on which side of the double bond it is preferentially formed depends, in the case of asymmetric alkenes, on the stabilization by different radicals. Markovnikov's rule offers a rule which of the products is preferred .
If the addition of a functional group is to take place on the less substituted carbon atom of the double bond, electrophilic substitution with acids is not possible. One possibility is hydroboration , in which the boron atom acts as an electrophile and therefore attacks the less substituted carbon atom in accordance with Markovnikov's rule. Other functional groups can then be formed in a further step by oxidation or halogenation .
While electrophilic addition occurs primarily with electron-rich alkenes and alkynes, nucleophilic addition plays an important role in carbon-heteroatom multiple bonds and, above all, their most important representative, the carbonyl group . This is often associated with an elimination, so that the carbonyl group is present again after the reaction. This can be done in the case of carboxylic acid derivatives such as carboxylic acid chlorides , esters or anhydrides which have a suitable leaving group on the carbonyl group. It is often referred to as the addition-elimination mechanism . This is often catalyzed by acids or bases which (in the case of acids) by attachment to the oxygen atom increase the electrophilicity of the carbonyl group or (in the case of bases) the nucleophilicity of the attacking nucleophile.
According to the principle of vinylogy, attack by a nucleophilic addition can also take place on the double bond of α, β-unsaturated carbonyl compounds such as ketones or esters . An important representative of this type of reaction is the Michael addition .
Like substitutions, additions can be triggered not only by nucleophiles and electrophiles, but also by radicals. As with radical substitution, radical addition also takes place in the form of a chain reaction. This reaction is the basis of radical polymerization .
Other organic reaction mechanisms
Rearrangements are reactions in which the atoms or molecular parts of an organic compound are retained but rearranged. These include hydride shift reactions such as the Wagner-Meerwein rearrangement , in which a carbocation is initially formed, which is then rearranged to a more stable carbocation by shifting a hydride ion. In most cases, however, rearrangements are associated with the breaking and re-formation of CC bonds. Typical examples of this are sigmatropic rearrangements such as the Cope rearrangement , in which one C — C bond is broken and another is formed in a cyclic reaction.
Like the sigmatropic rearrangements, cycloadditions also belong to the pericyclic reactions . In this reaction, a cyclic molecule is formed from several, usually two, double bonds containing molecules. The most important cycloaddition is the Diels-Alder reaction , a [4 + 2] cycloaddition in which a diene and an alkene (also known as a dienophile) react to form a cycloalkene.
In addition to the Diels-Alder reaction, there is also the [2 + 2] cycloaddition , in which two alkenes or other compounds with double bonds react with one another like ketones. Cycloadditions are also possible with 1,3-dipoles such as ozone , diazomethane or nitrile oxides . Whether and how a cycloaddition takes place depends on the arrangement of the p orbitals of the double bonds involved.
These have to stand against each other in such a way that orbitals with the same sign of the wave function overlap and can thus interact constructively and form the energetically more favorable single bonds. Cyclic reactions can be induced both thermally and photochemically by irradiation with light. Since during irradiation electrons are brought into orbitals that have a different arrangement and symmetry, photochemically different cycloadditions are possible than thermal. Diels-Alder reactions are thermal cycloadditions, while [2 + 2] cycloadditions have to be induced by irradiation.
The orbital arrangements limit the possible products and, in the case of stereoisomeric starting materials, also their stereoisomerism . How this takes place is described by the Woodward-Hoffmann rules .
Biochemical reactions
Enzymes are of central importance in biochemical reactions . These proteins usually specifically catalyze a single reaction so that reactions can be controlled very precisely. However, enzymes are also known which can catalytically accelerate several special functions. The reaction takes place in a small part of the enzyme, the active center , while the rest of the enzyme is mainly used for stabilization. The active center lies in a pit or groove in the enzyme. For the catalytic activity, among other things, bonds to the enzyme, the changed, hydrophobic , chemical environment and the spatial proximity of the reactants are responsible, while the special shape of the active center is responsible for the selectivity.
The totality of the biochemical reactions in the body is called metabolism . One of the most important mechanisms is the building metabolism , in which complex natural substances such as proteins or carbohydrates are synthesized from simple precursor substances in different processes controlled by DNA and enzymes, such as protein biosynthesis . In addition, there is the energy metabolism , through which the energy necessary for a reaction, e.g. the building metabolism , is provided with the help of chemical reactions. An important source of energy is glucose , which can be produced by plants during photosynthesis or is taken in with food. However, this cannot be used directly; instead, through cellular respiration and the respiratory chain with the help of oxygen, ATP is generated, which serves as an energy supplier for further reactions.
Technical application
Chemical reactions and their implementation are central to technical chemistry . They are used in large numbers to synthesize new compounds from naturally occurring raw materials such as petroleum , ores , air or renewable raw materials . Often, simple intermediate products are first synthesized, from which end products such as polymers , detergents , pesticides , pharmaceuticals or dyes are made. Technical reactions take place in reactors such as stirred tanks or flow tubes.
It is particularly important for technology to make the reaction process as economical as possible. These include minimal use of raw materials and energy, high reaction speeds and high yields with as few waste products as possible. The use of catalysts that both increase the reaction rate and reduce the energy consumption is therefore of great importance. In order to ensure small amounts of waste, reactions are often chosen in technical applications which have a high atom economy , i.e. in which a large part of the starting materials is found in the desired product.
observation
How chemical reactions can be observed and followed depends heavily on the speed of the reaction. In the case of slow reactions, samples can be taken and analyzed during the reaction. The concentrations of the individual ingredients of the reaction mixture are determined and the course of the concentration monitored during the reaction. If the concentrations no longer change after a while, the reaction is complete and equilibrium has been reached. To ensure that the reaction does not progress too much during the measurement, analysis methods that are quick and easy to carry out, such as thin-layer chromatography or mass spectrometry, are used. Continuous observation during the reaction is also possible using spectroscopic methods if, for example, the concentration of a colored substance in the mixture can be determined. If this is not possible, a special marker, such as a radioactive isotope , can be used, the concentration of which is then measured. This is used, for example, in scintigraphy for the observation of metabolic processes in which certain elements accumulate in individual organs. Under favorable conditions, surface reactions can be observed directly at the molecular level with a scanning tunneling microscope .
For detection reactions so-called play indicators an important role, which are substances that change, for example in color when a certain point of the reaction is reached. Acid-base indicators, which change their color as soon as a solution has been neutralized and the pH value changes from acidic to basic or vice versa, are known above all . Selective precipitation reactions can also be used to detect substances or, for example, in the cation separation process for separation prior to precise detection.
The faster a reaction occurs, the more difficult it becomes to observe. Ultrafast spectroscopy is used for kinetic investigations of fast reactions. With the help of femtosecond lasers, it enables a time resolution in the range of picoseconds or femtoseconds. In this way, short-lived transition states can also be observed during the reaction.
literature
- Entry on reaction. In: Römpp Online . Georg Thieme Verlag, accessed on June 20, 2014.
- Peter W. Atkins , Julio de Paula: Physical chemistry. 4th edition, Wiley-VCH, Weinheim 2006, ISBN 978-3-527-31546-8 .
- AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , pp. 186-258.
- Reinhard Brückner: reaction mechanisms. 3rd edition, Spektrum Akademischer Verlag, Munich 2004, ISBN 3-8274-1579-9 .
Web links
- Entry on chemical reaction . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.C01033 .
Individual evidence
- ↑ Jost Weyer: Newer interpretations of alchemy. In: Chemistry in Our Time. 1973, 7,6, pp. 177-181, doi: 10.1002 / ciuz.19730070604 .
- ^ William H. Brock: Viewegs Geschichte der Chemie . Vieweg, Braunschweig 1997, ISBN 3-540-67033-5 , pp. 34-55.
- ^ William H. Brock: Viewegs Geschichte der Chemie . Vieweg, Braunschweig 1997, ISBN 3-540-67033-5 , pp. 104-107.
- ↑ Entry on chemical reaction equation . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.C01034 .
- ^ EJ Corey: Robert Robinson Lecture. Retrosynthetic thinking — essentials and examples. In: Chem. Soc. Rev. 1988, 17, pp. 111-133, doi: 10.1039 / CS9881700111 .
- ↑ Entry on elementary reactions. In: Römpp Online . Georg Thieme Verlag, accessed on June 20, 2014.
- ↑ Peter W. Atkins, Julio de Paula: Physical chemistry. 4th edition, Wiley-VCH, Weinheim 2006, ISBN 978-3-527-31546-8 , pp. 106-108.
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 897.
- ↑ Peter W. Atkins, Julio de Paula: Physical chemistry. 4th edition, Wiley-VCH, Weinheim 2006, ISBN 978-3-527-31546-8 , p. 150.
- ↑ Peter W. Atkins, Julio de Paula: Physical chemistry. 4th edition, Wiley-VCH, Weinheim 2006, ISBN 978-3-527-31546-8 , p. 963.
- ^ A b A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , pp. 1380-1400.
- ↑ Entry on precipitation. In: Römpp Online . Georg Thieme Verlag, accessed on June 20, 2014.
- ^ Ralf Alsfasser, Erwin Riedel, C. Janiak, HJ Meyer: Modern inorganic chemistry. 3. Edition. de Gruyter, 2007, ISBN 978-3-11-019060-1 , p. 171.
- ↑ Peter W. Atkins, Julio de Paula: Physical chemistry. 4th edition, Wiley-VCH, Weinheim 2006, ISBN 978-3-527-31546-8 , pp. 937-950.
- ↑ Christoph Elschenbroich: Organometallchemie. 6th edition, Teubner Wiesbaden, 2008, ISBN 978-3-8351-0167-8 , p. 263.
- ↑ Reinhard Brückner : reaction mechanisms. 3rd edition, Spektrum Akademischer Verlag, Munich 2004, ISBN 3-8274-1579-9 , pp. 63-77.
- ↑ Reinhard Brückner: reaction mechanisms. 3rd edition, Spektrum Akademischer Verlag, Munich 2004, ISBN 3-8274-1579-9 , pp. 203-206.
- ↑ Reinhard Brückner: reaction mechanisms. 3rd edition, Spektrum Akademischer Verlag, Munich 2004, ISBN 3-8274-1579-9 , p. 16.
- ↑ Reinhard Brückner: reaction mechanisms. 3rd edition, Spektrum Akademischer Verlag, Munich 2004, ISBN 3-8274-1579-9 , p. 183.
- ↑ Reinhard Brückner: reaction mechanisms. 3rd edition, Spektrum Akademischer Verlag, Munich 2004, ISBN 3-8274-1579-9 , p. 192.
- ↑ Reinhard Brückner: reaction mechanisms. 3rd edition, Spektrum Akademischer Verlag, Munich 2004, ISBN 3-8274-1579-9 , p. 172.
- ↑ Reinhard Brückner: reaction mechanisms. 3rd edition, Spektrum Akademischer Verlag, Munich 2004, ISBN 3-8274-1579-9 , p. 125.
- ↑ Hans Peter Latscha, Uli Kazmaier, Helmut Alfons Klein: Organische Chemie: Chemie-Basiswissen II, Volume 2. 6th Edition, Springer, 2008, ISBN 978-3-540-77106-7 , p. 273.
- ↑ Reinhard Brückner: reaction mechanisms. 3rd edition, Spektrum Akademischer Verlag, Munich 2004, ISBN 3-8274-1579-9 , p. 580.
- ^ Manfred Lechner, Klaus Gehrke, Eckhard Nordmeier: Makromolekulare Chemie. 3rd edition, Birkhäuser, Basel 2003, ISBN 3-7643-6952-3 , pp. 53–65.
- ↑ Eberhard Breitmaier, Günther Jung: Organic chemistry. 5th edition, Thieme, Stuttgart 2005, ISBN 3-13-541505-8 , pp. 447-453.
- ↑ Reinhard Brückner: reaction mechanisms. 3rd edition, Spektrum Akademischer Verlag, Munich 2004, ISBN 3-8274-1579-9 , pp. 637–647.
- ^ RB Woodward, Roald Hoffmann: Stereochemistry of Electrocyclic Reactions. In: J. Am. Chem. Soc. 1965, 87, 2, pp. 395-397, doi: 10.1021 / ja01080a054 .
- ^ Peter Karlson, Detlef Doenecke, Jan Koolman, Georg Fuchs, Wolfgang Gerok: Karlsons Biochemie und Pathobiochemie. 16th edition, Georg Thieme Verlag, 2005, ISBN 978-3-13-357815-8 , pp. 55-56.
- ^ Gerhard Emig, Elias Klemm: Technical Chemistry. 5th edition, Springer, 2005, ISBN 978-3-540-23452-4 , pp. 33-34.
- ^ Barry Trost : The atom economy - a search for synthetic efficiency. In: Science . 1991, 254, pp. 1471-1477, doi: 10.1126 / science.1962206 .
- ↑ Thomas Waldmann, Daniela Künzel, Harry E. Hoster, Axel Groß, R. Jürgen Behm: Oxidation of an Organic Adlayer: A Bird's Eye View. In: Journal of the American Chemical Society. 134, No. 21, 2012, pp. 8817-8822, doi: 10.1021 / ja302593v .
- ↑ Peter W. Atkins, Julio de Paula: Physical chemistry. 4th edition, Wiley-VCH, Weinheim 2006, ISBN 978-3-527-31546-8 , p. 987.










![{\ displaystyle v = - {\ frac {\ mathrm {d} [\ mathrm {A}]} {\ mathrm {d} t}} = k \ cdot [\ mathrm {A}]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/02e857d43648c97f61048ef4d2b8d945278946db)
![\ mathrm {[A]} _ {t} = \ mathrm {[A]} _ {0} \ cdot e ^ {- k \ cdot t}](https://wikimedia.org/api/rest_v1/media/math/render/svg/896c5da2771260d7b5dbd5f28a9406cda180e268)