Cope rearrangement
The Cope rearrangement is a reaction from the field of organic chemistry that goes back to the American chemist Arthur C. Cope (1909–1966). It is the first solid-phase synthesis to be discovered in organic chemistry. It is a reversible thermal isomerization of 1,5-dienes and belongs to the pericyclic reactions . The Cope rearrangement is a sigmatropic [3,3] shift.
Examples
In Fig. 1 the reaction is illustrated using the example of 1,5-hexadiene . The Cope rearrangement of unsubstituted 1,5-hexadiene yields 1,5-hexadiene again. This is an example of a degenerate reaction in which the starting material and product are identical.
In Fig. 2, the rearrangement of the meso form of a 3,4-disubstituted 1,5-hexadiene is described; Fig. 3 shows the rearrangement of the racemate of a 3,4-disubstituted 1,5-hexadiene. The reaction is stereospecific and stereoselective. Examples of the synthesis of large rings can be found in Fig. 4 and Fig. 5.
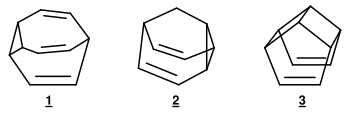
Further compounds that show the phenomenon of the degenerate Cope rearrangement are 3,4-homotropilids (Fig. 6), the bridged homotropilid derivatives Bullvalen and Barbaralan , and hypostrophs (Fig. 7). These molecules with fluctuating bonds are called valence tautomers .
Similar reactions
- Claisen rearrangement (Oxa-Cope rearrangement)
Individual evidence
- ↑ a b c d e László Kürti and Barbara Czakó .: Strategic Applications of Named Reactions in Organic Synthesis: Background and Detailed Mechanisms , Elsevier Academic Press, 2005, ISBN 978-0-12-429785-2 , pp. 98-99.
- ^ VK Ahluwalia: Strategies for Green Organic Synthesis . 1st edition. Ane Books Pvt. Ltd., New Delhi 2012, ISBN 978-1-4398-7050-1 , pp. 8 .
- ^ Hans Rudolf Christen: Fundamentals of Organic Chemistry , 5th edition, 1982, p. 575 ff, Otto Salle Verlag, Frankfurt, ISBN 3-7935-5395-7 .
- ↑ Eberhard wide Maier, Günther Jung: Organic Chemistry: Fundamentals, classes, reactions, concepts, molecular structure , p 452 Publisher: Thieme; 6th edition, 2009, ISBN 978-3-13-541506-2 .
further reading
- Arthur C. Cope, Elizabeth M. Hardy: The Introduction of Substituted Vinyl Groups. V. A Rearrangement Involving the Migration of an Allyl Group in a Three-Carbon System 1. In: Journal of the American Chemical Society. 62, No. 2, 1940, pp. 441-444, doi: 10.1021 / ja01859a055 .
- Ian Fleming: Frontier Orbitals and Reactions of Organic Compounds. (= Frontier orbitals and organic chemical reactions. ) 1st edition, corrected reprint, VCH, Weinheim 1988, ISBN 3-527-25792-6 .
- TH Lowry, KS Richardson Mechanisms and Theory in Organic Chemistry. Verlag Chemie, Basel 1980, ISBN 3-527-25795-0 , p. 735 ff.