Bullvalen
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||
| General | |||||||||||||
| Surname | Bullvalen | ||||||||||||
| other names |
Tricyclo [3.3.2.0 2,8 ] deca-3,6,9-triene ( IUPAC ) |
||||||||||||
| Molecular formula | C 10 H 10 | ||||||||||||
| Brief description |
crystalline solid |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 130.19 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| Melting point |
96 ° C |
||||||||||||
| boiling point |
from 400 ° C: decomposes to naphthalene and hydrogen |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
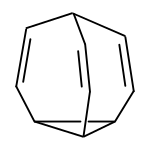
Bullvalene is an unsaturated policyclischer , bridged hydrocarbon with a cage structure , which despite a cyclopropane - structural element is extremely stable.
History and name
The origin of the name Bullvalen is not known for certain. Possibly it is based on the nickname of William "Bull" Doering , who in 1963, together with Wolfgang Richard Roth, predicted the properties of the molecule on the basis of theoretical calculations, as well as the property of the molecule to exist in very many different valence tautomeric forms. The first synthesis succeeded Gerhard Schröder in the same year by photolysis of a dimeric cyclooctatetraene with elimination of benzene .
presentation
Bullvalene can be obtained synthetically from cyclooctatetraene . A mixture of the two dimeric C 16 H 16 compounds 2 and 3 is obtained by heating cyclooctatetraene 1 . From the pentacyclic compound 2 , which has a homotropilid structural element, Bullvalene 4 is obtained photochemically by UV irradiation with elimination of benzene 5 .
Properties and reactions
Bullvalen forms colorless crystals with a melting point of 96 ° C. Only at 400 ° C does the substance break down into naphthalene and hydrogen. The compound enters into the expected addition reactions by consuming 4 moles of bromine or hydrogen for three double bonds and the cyclopropane ring. Substituted bullvalene derivatives are easily accessible by repeated bromination and subsequent dehydrobromination . The bromobullvalenes, in turn, can be converted into aryl, alkyl or fluobullvalenes by known substitution reactions .
structure
In Bullvalen, the three carbon atoms of a cyclopropane ring are linked in a star shape to a methine residue via three vinylene bridges to form a surprisingly stable tricyclic structure.
The reason lies in the stabilization of the system through valence isomerism , in this case through a [3,3] sigmatropic rearrangement, the so-called Cope rearrangement . There are three possible Cope systems in a discrete bullvalene molecule. During the Cope rearrangement in Bullvalene, the cyclopropane ring breaks through a six-membered transition state and a new cyclopropane ring is formed when the double bonds are shifted. Due to the specific structure of the bullvalene, a bullvalene molecule emerges from this rearrangement, only the carbon atoms individually take up different positions in the new molecule. This phenomenon is also known as the degenerate Cope rearrangement
From 100 ° C onwards, the Cope rearrangements between the more than 1.2 million valence isomers (exactly 10! / 3 = 1,209,600 possibilities) take place so quickly that the 1 H NMR spectrum of the hydrogen atoms only has a single, sharp signal at 4.2 ppm. This means that at this temperature all hydrogen atoms are equivalent and led to the expression of the "fluctuating structure". This assumption could be proven by X-ray structure analysis at room temperature and neutron diffraction experiments at 100 K.
Individual evidence
- ↑ a b c Ludwig Bergmann, Clemens Schaefer: Textbook of Experimentalphysik: Vol. 6 Solid , p. 196, de Gruyter Verlag, 1999
- ↑ Entry on Bullvalen. In: Römpp Online . Georg Thieme Verlag, accessed on July 7, 2014.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ WVE Doering, WR Roth: Thermal rearrangement reactions . In: Angewandte Chemie . tape 75 , no. 1 , January 7, 1963, p. 27 , doi : 10.1002 / anie.19630750106 .
- ^ Addison Ault: The Bullvalene Story. The Conception of Bullvalene, a Molecule That Has No Permanent Structure . In: Journal of Chemical Education . tape 78 , no. 7 , July 2001, p. 924 , doi : 10.1021 / ed078p924 .
- ↑ Gerhard Schröder: The properties of two dimeric cyclooctatetraene from melting point 53 and 76 ° . In: Chemical Reports . tape 97 , no. November 11 , 1964, pp. 3131 , doi : 10.1002 / cber.19640971124 .
- ↑ Gerhard Schröder: Synthesis and properties of tricyclo [3.3.2.0 4.6 ] decatrien- (2.7.9) 2.3) (Bullvalen) . In: Chemical Reports . tape 97 , no. November 11 , 1964, pp. 3140 , doi : 10.1002 / cber.19640971125 .
- ↑ Detlev Wendisch: Methods for the production and conversion of carbocyclic three-ring systems in: Houben-Weyl, Methods of Organic Chemistry Vol. IV / 3, 4th Edition
- ↑ Martin Saunders: Measurement of the rate of rearrangement of bullvalenes . In: Tetrahedron Letters . tape 4 , no. 25 , 1963, pp. 1699-1702 , doi : 10.1016 / S0040-4039 (01) 90897-4 .
- ↑ Chemgapedia.de: Pericyclic reactions: Sigmatropic rearrangements

