Cyclooctatetraene
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Cyclooctatetraene | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 8 H 8 | |||||||||||||||
| Brief description |
yellow liquid with an unpleasant odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 104.15 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.93 g cm −3 |
|||||||||||||||
| Melting point |
0 ° C |
|||||||||||||||
| boiling point |
142-143 ° C |
|||||||||||||||
| Vapor pressure |
10.5 h Pa (20 ° C) |
|||||||||||||||
| solubility |
almost insoluble in water |
|||||||||||||||
| Refractive index |
1.5381 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Cyclooctatetraene (COT) is an organic - chemical compound from the group of cyclic hydrocarbons . The compound with the empirical formula C 8 H 8 has four conjugated C = C double bonds .
In contrast to benzene (C 6 H 6 ), COT is not one of the aromatic hydrocarbons because, due to the number of its π electrons, it does not conform to Hückel's rule and is also not planar, but rather has a tub shape .
Three-dimensional framework formula in all - ( Z ) - configuration with visible boat conformation
Thus, cyclooctatetraene is neither an aromatic nor an anti- aromatic . Due to the lack of aromatic stabilization, it is more comparable to the usual polyenes , however, due to the deformation of the bond angle caused by the ring strain , it is of increased reactivity.
Like 1,5-cyclooctadiene (COD), the tub-shaped COT molecule can form metal complexes as a chelating ligand . By absorbing two electrons, e.g. B. by transferring a metal , the COT becomes the planar, aromatic cyclooctatetraenyl anion C 8 H 8 2− with ten π electrons and thus fulfills the Hückel rule. A well-known example of a complex with planar cyclooctatetraenyl ligands is uranocene .
presentation
- COT was first synthesized from pseudopelletierin in 1905 by Richard Willstätter .
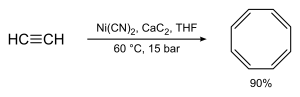
- The most common synthesis of COT is based on a Walter Reppe method by cyclotetramerizing ethyne .
- Another synthesis is based on cubane . In the presence of rhodium catalysts, the syn - tricyclooctadiene is first formed, which can then be thermally converted at 50-60 ° C. to cyclooctatetraene.
properties
By heating cyclooctatetraene 1 to 100 ° C., a mixture of the two dimeric C 16 H 16 compounds 2 and 3 is obtained . The dimer 2 (melting point 53 ° C) is created by a Diels-Alder reaction of two molecules of cyclooctatetraene ( 1 ). This reaction is partially reversible at high temperature. In a second step, 2 rearranges into dimer 3 (melting point 76 ° C.).
From the pentacyclic compound 3 , which has a homotropilid structural element , the C 10 H 10 hydrocarbon Bullvalene 4 is obtained by UV irradiation with elimination of benzene 5 .
Cyclooctatetraene is used in the synthesis of suberic acid and cyclooctane .
Individual evidence
- ↑ a b c d Datasheet Cyclooctatetraen from AlfaAesar, accessed on January 7, 2008 ( PDF )(JavaScript required) .
- ↑ a b Datasheet Cyclooctatetraene from Acros, accessed on February 19, 2010.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-130.
- ↑ a b Datasheet Cyclooctatetraene from Sigma-Aldrich , accessed on November 7, 2016 ( PDF ).
- ↑ Frank-Gerrit Klärner: How anti-aromatic is planar cyclooctatetraene? In: Angewandte Chemie . tape 113 , no. 21 , 2001, p. 4099-4103 , doi : 10.1002 / 1521-3757 (20011105) 113: 21 <4099 :: AID-ANGE4099> 3.0.CO; 2-1 .
- ↑ Richard Willstätter, Ernst Waser: About Cyclo-octatetraen . In: Reports of the German Chemical Society . tape 44 , no. 3 , 1911, pp. 3423-3445 , doi : 10.1002 / cber.191104403216 .
- ^ Walter Reppe, Otto Schlichting, Karl Klager, Tim Toepel: Cyclizing Polymerization of Acetylene I. About Cyclooctatetraen . In: Justus Liebig's Annals of Chemistry . tape 560 , no. 1 , 1948, p. 1-92 , doi : 10.1002 / jlac.19485600102 .
- ↑ L. Cessar, PE Eaton, J. Halpern: Catalysis of symmetry-restricted reactions by transition metal compounds. Valence isomerization of cubane , In J. Am. Chem. Soc. 92, 1972, pp. 3515-3518. doi : 10.1021 / ja00714a075 .
- ↑ Gerhard Schröder: The properties of two dimeric cyclooctatetraene from melting point 53 and 76 . In: Chemical Reports . tape 97 , no. November 11 , 1964, pp. 3131 , doi : 10.1002 / cber.19640971124 .
- ↑ Gerhard Schröder: Synthesis and properties of tricyclo [3.3.2.0 4.6 ] decatrien- (2.7.9) 2.3) (Bullvalen) . In: Chemical Reports . tape 97 , no. November 11 , 1964, pp. 3140 , doi : 10.1002 / cber.19640971125 .
- ↑ Entry on cyclooctatetraene. In: Römpp Online . Georg Thieme Verlag, accessed on September 27, 2019.