syn - anti notation
syn and anti is used in organic chemistry to
- to name the relative position of two substituents in bridged bicyclic hydrocarbons and
- to clearly describe the course of the reaction in addition reactions.
syn - and anti - in bicyclic hydrocarbons
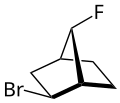
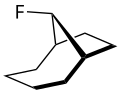
The syn - anti - isomerism describes the relative position of a substituent which is located at the third bridge of a bicycle. This substituent can occupy two isomeric positions, which are identified with the descriptors syn - or anti -:
As syn is used to refer that position, in which this substituent is on the side of the first bridge. The opposite position of the substituent is called anti .
The three bridges of a bicycle are arranged as follows:
Based on the two bridgehead atoms , the length of each individual bridge is first determined. They are then ranked according to the length of the bridge, the longest being the first and the shortest being the third. If two bridges are of the same length, the higher substituted bridge is ranked first.
syn - and anti - in addition reactions
In addition reactions, the syn - anti notation describes the entry point of the two substituents to be added.
As syn referred to those addition reaction occur at the both substituents on the same side of the alkene or alkyne. As anti refers to those addition reaction occurs in each substituent on another side of the alkene or alkyne.
In the case of addition reactions on alkynes , the syn addition leads to the formation of the corresponding ( Z ) isomers (also outdated: cis isomers ), and the anti addition leads to the formation of the corresponding ( E ) isomers ( trans isomers ).

Substituted cycloalkanes result from addition reactions on the double bond of cycloalkenes:

The addition reactions to the double bond of alkenes lead to the corresponding substituted alkanes . Thus on a CC single bond, a statement of syn - or anti - conformation can be taken, the other positions have in the formation of chiral be substituted correspondingly different products so that a chiral product is obtained. Otherwise, the reaction products could be converted into one another by rotation about the CC single bond. A syn / anti distinction would therefore be unnecessary.
syn - and anti - in elimination reactions
In organic chemistry, elimination reactions turn a CC single bond into a C = C double bond. Depending on the structure of the starting material, cis or trans stereoisomeric alkenes can be formed. The reaction mechanism of the elimination reaction also has an influence:
- in a two-stage elimination reaction according to the E1 mechanism, the stereochemistry of the alkene is only determined by the splitting off of a proton from the carbenium ion that has formed in the meantime.
- in a two-stage elimination reaction according to the E1cb mechanism, the stereochemistry of the alkene is only determined by the elimination of a leaving group X from the carbanion that has formed in the meantime.

- in an elimination reaction according to the E2 mechanism, no intermediate is formed, two single bonds (starting from adjacent sp 3 carbon atoms) are broken and the C = C double bond of an alkene is formed synchronously. The two sp 3 carbon atoms result in sp 2 hybridized carbon atoms. The σ-bonds to be broken must lie in one plane. This is only possible with a staggered anti- arrangement or with an ecliptic syn- arrangement. In the anti -elimination the σ-bonds are anti- periplanar to one another.
See also
Individual evidence
- ↑ Entry on endo, exo, syn, anti . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.E02094 Version: 2.3.3.
- ↑ Chemgapedia: Glossary of syn / anti notation
- ^ Joachim Buddrus: Fundamentals of Organic Chemistry , 4th Edition, de Gruyter Verlag, Berlin, 2011, pp. 286–289, ISBN 978-3-11-024894-4 .
- ^ Ulrich Lüning: Organic reactions , Spectrum Akademischer Verlag Heidelberg, 3rd edition, 2010, pp. 59–61, ISBN 978-3-8274-2478-5 .