cis - trans isomerism
The cis - trans isomerism or ( Z ) - ( E ) isomerism describes a special form of configuration isomerism in chemistry in which the molecules differ only in terms of the relative position of two substituents with respect to a reference plane. In contrast to conformers , cis-trans isomers can only be converted into one another by breaking a bond. If there are more than two substituents, those two are considered which have the highest priority according to the Cahn-Ingold-Prelog convention .
- Of a cis - or ( Z ) arrangement is when both substituents on the same side of the reference plane ( z are ogether).
- By a trans - and ( E ) device is used when the two substituents on opposite (sides of the reference plane e ntgegen) are located.
A reference plane can be, for example, a double bond or a ring system . cis and trans isomers differ in their chemical and physical properties such as melting temperature , boiling temperature , enthalpy of binding . cis - and trans - or ( Z ) - and ( E ) - themselves are referred to as descriptors and are placed in front of the systematic substance name.
cis - trans isomerism in double bonds
cis - trans isomerism occurs when both atoms of a double bond have different substituents:
- If both substituents are on the same side , one speaks of a cis or ( Z ) isomer.
- If the two substituents are on opposite sides of the double bond, one speaks of a trans or ( E ) isomer.
An example of ( Z ) - ( E ) isomerism is the isomer pair ( Z ) -butenedioic acid ( maleic acid ) and ( E ) -butenedioic acid ( fumaric acid ).
If a hydrogen atom and a larger substituent are attached to each of the two carbon atoms of a double bond ( vicinal double bonds), the assignment of cis and trans is clear and these traditional nomenclature names can be used. If, on the other hand, there are more substituents, the ( E / Z ) nomenclature must be used, for which a clear priority order has been defined via the sequence rule.
cis - trans isomerism in ring systems
There is also cis - trans isomerism in cyclic compounds, for example in monocycles that have substituents on different carbon atoms, or in bicycles. To name ring systems according to IUPAC rules, the prefixes " cis -" and " trans -" are placed in front of the connection name in italics and used for alphabetical classification only if names are identical.
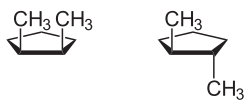
An example of a monocyclic compound with cis - trans isomerism is 1,2-dimethylcyclopentane : The two methyl substituents can either be on the same side of the molecular plane ( cis form) or on opposite sides ( trans form).
With 6-rings, as with 1,2-dimethylcyclohexane , the cis and trans isomers are difficult to see because the compounds should not be viewed as planar rings. Compounds of this type are mostly in the energetically preferred chair conformation . Since there are two chair conformations due to ring inversion , trans -1,2-dimethylcyclohexane and cis -1,2-dimethylcyclohexane , for example, can not be recognized directly. In trans -1,2-dimethylcyclohexane, both methyl groups are either equatorial or axial . In the cis forms, one methyl group is located equatorially, the other axially.

|

|
| (1a) and (1b) chair conformations of trans -1,2-dimethylcyclohexane; (2a) and (2b) Chair conformations of cis -1,2-dimethylcyclohexane. | Positions in the chair conformation of cyclohexane . The hydrogen atoms in the axial position are marked in red and trans -positioned to one another . The hydrogen atoms in the equatorial position are marked in blue and are also in the trans position to one another , but this is more difficult to see. |
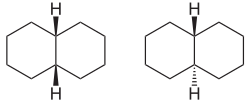
An example of a bicyclic compound is decalin , as the two six-membered rings can be linked differently: The two hydrogen atoms at the connection points can either be on the same side of the decalin molecule ( cis form) or on opposite sides ( trans form).
Mutual transformation
| Equilibrium catalyzed by a peptidyl-prolyl- cis - trans -isomerase (PPIase): Without enzymatic catalysis, the rotation around the blue- marked axis of the peptide bond between the N -terminus of L - proline and the C -terminus of another amino acid takes place only slowly . |
In the case of a double bond, the cis and trans isomers can be converted into one another ( cis - trans interconversion). This requires a temporary breakdown of the π bond, which is usually done photochemically or at higher temperatures. The process of photochemical interconversion takes place here by means of electronic excitation into the singlet spin state. The non-twisted molecular structure is sterically as well as energetically disadvantaged. This is followed by a spin reversal of the electron excited from the HOMO (now SOMO; single occupied molecular orbital) into the triplet state, which is in equilibrium with the cis - trans recombination. When the reaction temperature is increased, the thermodynamic trans product is obtained here.
cis - trans conversions are e.g. B. a central component of the visual process (see retinal ).
The amide bond (peptide bond) present between the N terminus of L -proline and the C terminus of another amino acid in peptides can be in the trans as well as in the cis configuration, since this bond has a certain double bond character. A change between the two configurations takes place only slowly, but can be enzymatically catalyzed by a peptidyl prolyl cis - trans isomerase.
See also
Individual evidence
- ↑ D. Hellwinkel: The systematic nomenclature of organic chemistry. 5th edition, Springer, 2005, ISBN 3-540-26411-6 , p. 188.
- ^ Albert Gossauer: Structure and reactivity of biomolecules , Verlag Helvetica Chimica Acta, Zurich, 2006, p. 102, ISBN 978-3-906390-29-1 .
- ↑ Paula Yurkanis Bruice , Oliver Reiser : Organic Chemistry Pearson Education, Munich 2007, ISBN 9783827371904 , pp 159th
- ↑ Michael W. Tausch : Photochemical cis-trans isomerizations Der Mathematische und Naturwissenschaftlichen Studium, (MNU), 40:92, URL: /fileadmin/Chemie/chemied...ublications/mnu_40_87.pdf
literature
- Entry on cis-trans isomerism. In: Römpp Online . Georg Thieme Verlag, accessed on June 13, 2014.
- Jerry March: Advanced Organic Chemistry. Reactions, Mechanisms, and Structure. 3rd edition, John Wiley & Sons, New York 1985.