Substance property
A substance property is a substance-specific, characteristic quantity that characterizes a pure substance . It can be perceived with the senses (e.g. the smell) or can only be recorded with measuring devices (e.g. the density of a substance).
Each basic substance is characterized by a unique combination of substance properties that can be used to identify it. Two substances cannot be the same in all properties.
Mixtures of substances differ from pure substances in that their properties depend on their components and their mixing ratios.
Physical substance properties
Physical substance properties are the substance-specific values that can be assigned to a physical quantity through measurements and experiments . During the measurement, a physical property of the measurement object, in contrast to the chemical properties, is not changed.
The physical properties include:
- Color or absorption and emission spectrum
- density
- Thermal conductivity
- Electric conductivity
- magnetic permeability (magnetic conductivity) and remanence (magnetizability)
- Physical state (solid, liquid, gaseous) at a certain temperature
- Melting temperature or softening range
- Boiling temperature
- optical activity
- solubility
- viscosity
- Enthalpy of vaporization , enthalpy of fusion
- Surface tension
- Critical temperature , critical pressure , critical density
- Heat capacity
- Speed of sound
- Nuclear magnetic resonance
Especially for solids :
- Deformability
- Extensibility
- Surfaces gloss
- hardness
- Temperatures and enthalpies of the transition between solid modifications
Chemical properties of substances

The chemical properties of the substance include:
- Reactivity to various other materials (e.g., to. Oxygen : flammability , but also towards important reagents, such as water , acids , bases , metals, saline solutions, chlorine gas, sulfur , detection reagents, etc.)
- Corrosion resistance (to water, moist air, electrolyte solutions)
- Electronegativity (for elements)
- Enthalpy of formation , enthalpy of combustion , Gibbs free enthalpy of formation
- Acid constant K S or base constant K B
Physiological properties
Physiological properties are physical and chemical properties of substances from the aspect of perceptibility or the effects on the environment.
The physiological properties of the substance include:
- odor
- taste
- metabolism
- Toxicity , ecotoxicity (environmental damage) and similar biological effects
- Absorbability
Use in chemistry and technology
Materials
The respective material properties make a material technically usable as a material (iron as hard and tough for tools of all kinds, glass as malleable and chemically stable for vessels, as transparent for windows, ceramics as heat-resistant , etc.). Here one speaks specifically of material properties , the central research area of materials science .
Separation of mixtures of substances
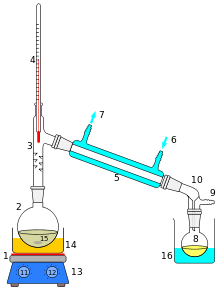
1 : Heating source
2 : Distillation flask
3 : Distillation attachment
4 : Thermometer
5 : Liebig condenser
6 : Cooling water inlet
7 : Cooling water outlet
8 : Round-bottomed flask for the distillate
9 : Pressure equalization
10 : Advance
11 : bath temperature controller
12 : knob speed magnetic stirrer
13 : magnetic stirrer, heating plate
14 : heating bath (water bath, oil bath)
16 : magnetic stirrer, boiling chips
16 : cooling bath, possibly ice bath.
Using different material properties, material mixtures can be separated into their individual components ( material separation process , separation technology):
- In a distillation z. B. a mixture of several liquids separated by heating the mixture above the boiling point of a component. This then boils, so that the less volatile part remains. The vapor is cooled in the cooler below the boiling / condensation temperature of the more volatile component. This is then collected as distillate or condensate in the receiver.
- In fractional crystallization , a mixture of solids is dissolved in a suitable solvent and a supersaturated solution is produced by evaporation or evaporation, from which a solid of the mixture can preferably be crystallized out and isolated by filtration.
- An iron powder / sand mixture, on the other hand, can be separated by using the magnetism of iron (a physical property) - or by dissolving the mixture in acid, which etches the iron, but leaves the sand undissolved and therefore filterable (chemical property).
Purity control
In a pharmaceutical or chemical laboratory newly produced preparation , a chemical substance to further characterize and control its purity and quality, we examined its characteristics. Often, highly developed analytical techniques are used to control the quality of chemical and pharmaceutical products. B. NMR spectroscopy .
Identification of substances
To identify organic substances in industrial incoming goods inspection , the infrared spectrum (IR spectrum) is often recorded and compared with the IR reference spectrum of a standard. If the IR spectra - especially in the fingerprint area - are identical, this is very good proof of identity.
Classification of substances
In analytics , substances in an unknown sample are identified and classified based on their substance properties. Certain properties also characterize certain groups of substances such as B. the substance classes of metals, salts, noble gases, halogens or organic substances :
- All metals conduct electricity and heat well, are easily deformable, have a surface gloss in their pure state (but appear black in the finely divided state; subgroups: alkali metals , precious metals , non-ferrous metals, etc.).
- Non-metals (molecular substances such as halogens and hydrocarbons ) hardly conduct electricity ( insulators ), are mostly brittle in the solid state and have low boiling points (unless they are diamond or plastic-like: Macromolecular substances often have high melting and boiling temperatures, but usually decompose at relatively low temperatures, examples: plastics , proteins , polysaccharides , DNA ).
- Salt-like substances have relatively high melting and boiling temperatures, conduct electricity as melts or solutions and are crystalline and brittle.
Web links
Individual evidence
- ↑ Atkins, PW & Beran, JA: Chemistry - Everything . VCH, Weinheim 1996.
- ^ Siegfried Hauptmann : Organic Chemistry , 2nd reviewed edition, VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig, 1985, pp. 43–45, ISBN 3-342-00280-8 .
