metabolism
| Parent |
| Biological process |
| Subordinate |
|
Biosynthesis catabolism transport |
| Gene Ontology |
|---|
| QuickGO |
As metabolism or metabolism ( ancient Greek μεταβολισμός metabolismós , German , metabolism ' , with Latin ending -US ) is defined as the entire chemical and physical processes of conversion of chemical substances or substrates (eg. Foodstuffs and oxygen) in the intermediate products ( metabolites ), and End products in the organism of living things . These biochemical processes serve to build up, break down and replace or maintain the body substance ( building metabolism) as well as the generation of energy for energy-consuming activities ( energy metabolism ) and thus the maintenance of body functions and thus of life. Enzymes that accelerate and direct ( catalyze ) chemical reactions are essential for the metabolism .
If foreign substances absorbed from the outside are converted, one also speaks of foreign substance metabolism . The conversion of substances foreign to the organism into substances inherent in the organism is called assimilation . The opposite is dissimilation (breakdown of the organism's own substances). The metabolism also includes the conversion of harmful substances into excretable substances ( biotransformation ).
Metabolic processes are primarily researched in biochemistry. They are of great importance in medicine and physiology (see also metabolic disorder ). But they can also be interpreted physically , as an exchange of free energy for order: living beings increase order and consume energy in the process. Entropy (disorder) decreases in the organism and increases in the environment.
Catabolic and anabolic metabolism
The entire metabolism can be divided into catabolic reactions, which deliver energy through the breakdown of chemically complex nutrients into simpler substances ( catabolism ), and anabolic reactions, which build up the body's own substances from simple components while consuming energy ( anabolism ).
Catabolism and anabolism have a common interface: In the intermediate metabolism , relatively simple molecules are converted which can be provided as intermediate products ( metabolites ) by both the catabolic and anabolic metabolism.
| Some metabolic pathways and their links. (Substances to click.) |
Metabolic pathways
The metabolism is a complex network of individual reactions. Groups of reactions that follow one another are called metabolic pathways . These can be linear (e.g. glycolysis ) or cyclical (e.g. citric acid cycle ).
Most metabolic pathways are amphibolic, which means that they run in different steps catabolically and anabolically. Even if many individual steps are reversible , the entire metabolic pathway is always irreversible , since at least one reaction step only proceeds in an anabolic or catabolic direction.
Metabolic rate
The speed at which energy is provided by the energy metabolism is called the metabolic rate or metabolism rate . It is defined as the organism's energy expenditure per unit of time. The basal metabolic rate is the energy expenditure at complete rest; especially in humans one speaks of the basal metabolic rate .
Reaction types
Enzymatically catalyzed reactions
According to the IUPAC / IUBMB enzyme classification system, there are six main groups of enzyme reactions. Thereby, also the metabolism, in the reactions by enzymes catalyzes will be also divided into six groups divided by reactions, namely in redox reactions , group transfer reactions, hydrolysis reactions, lyase reactions ( addition , hydration ), isomerization reactions and ligation reactions.
Electric charge and cation current
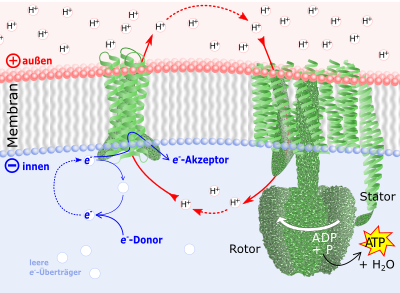
Green: membrane oxidoreductase (left) and ATP synthase (right). Back-flowing cations set the lower part of the ATP synthase in rotation. Water is "squeezed out" of the phosphate and ADP absorbed there . The finished ATP is released at the stator, which is anchored in the membrane .
Red: ion transport through membrane enzymes. An oxidoreductase (left) pumps H + ions from the inside through the membrane to the outside and increases the electrical charge of the membrane and the proton gradient .
Blue: electron transfer . The energy for the oxidoreductases comes from a flow of electrons from e - donors (such as e.g. sugars) to an e - acceptor (mediated by coenzymes such as NADH ), which act as e - carriers.
Cell and biomembrane are by no means just “bags” in which enzymes and their reaction partners can move freely. Rather, they form an impenetrable barrier for charged molecules ( ions ). In the membranes, however , there are membrane proteins that selectively allow ions to pass through the membrane or even actively transport them. By exporting cations , the latter ensure that the membranes are electrically charged positively on the outside and negatively charged on the inside ( membrane potential ). There is energy in this charge. It becomes free for a number of transport and movement processes when the cations flow back.
A stream of positively charged particles constantly flows through the membranes of practically all living cells.
This current is driven by exergonic chemical reactions. Nerve cells continuously consume ATP in order to maintain their membrane potential. This is done there by ATPases , which act as sodium-potassium pumps .
The regeneration of used ATP is based almost exclusively on the use of the membrane potential by the enzyme ATP synthase in almost all living things (Fig. 1). In specialized organelles (the mitochondria ) of animals and plants as well as in almost all archaea and bacteria , oxidoreductases function as cation pumps, which maintain the membrane potential. In humans, these are the enzymes in the respiratory chain . Plants also use their photosynthesis apparatus in the chloroplasts for this .
transport
The transport of substances by transport proteins can take place within cell compartments , outside cells, or across compartment boundaries ( biomembrane ). It may be pure diffusion processes, facilitated diffusion or active, ATP -consuming membrane transport act.
With the Transporter Classification Database (TCDB), a classification of the transport proteins sanctioned by the IUBMB is available, which, in addition to its function, is based on the origin of the proteins. With this definition of transport, however, all proteins that only bind substances temporarily and are transported themselves during this time (for example with the bloodstream ) are not recorded. The main groups in the TCDB are porins and ion channels , potential-driven transporters, primarily active transporters, phosphotransferases , transmembrane electron carriers , auxiliary transporters and others.
Metabolic types in different groups of living things
Plants , algae , some bacteria and archaea operate photosynthesis . They use the energy of light to convert carbon dioxide (in land plants from the air ), water and other raw materials into the body's own material. This either serves the further development and growth of the organism immediately , or it serves as a storage material, such as carbohydrates (see also Calvin cycle ). The reserve materials can later be processed in the building metabolism or in the energy metabolism. Secondary plant substances are chemical compounds that are produced by plants, but are neither required for building metabolism nor for energy metabolism.
Animals metabolize (metabolize) other organisms or their storage materials (such as carbohydrates, proteins or fats ) during their digestion , see also chemotrophy .
There are other types of metabolism in microorganisms .
In addition, the advisory literature on nutrition and diets occasionally mentions different “metabolic types” in humans, see Metabolic Typing . Individual characteristics within individual species have nothing to do with the above-mentioned classification of living beings . People differ in features of their metabolism as well as other features. However, the doctrine of certain "types" is considered speculative and unscientific.
See also
- Amino acid metabolism , glucose metabolism , iron metabolism
- Starvation metabolism
- Metabolome , tumor metabolome
- Pasteur effect (anaerobic metabolism of D- glucose)
- Trace element
- endocrinology
literature
- Pschyrembel Clinical Dictionary. Founded by Willibald Pschyrembel. Edited by the publisher's dictionary editor. 255th edition. De Gruyter, Berlin 1986, p. 1601 f.
Web links
- Biochemistry of Metabolism - comprehensive information on the biochemical aspects of metabolism
- SuperCYP: Database cytochrome metabolism (Engl.)
- Roche's Biochemical Pathways as a "map" for navigation
Individual evidence
- ↑ IUPAC nomenclature enzyme Recommendation: Recommendations of the Nomenclature Committee of the International Union of Biochemistry and Molecular Biology on the Nomenclature and Classification of Enzymes by the Reactions They Catalyze. As of May 24, 2013, accessed May 26, 2013.

