Membrane transport
In biology, membrane transport is understood to mean the transport of different substances through a biomembrane . If parts of the membrane itself are displaced at the same time, this is sometimes referred to separately as membrane flow .
The area enclosed by a biomembrane (for example the cytoplasm of a cell ) creates a largely controlled region. The shielding of the inside from the outside world enables the cell to build and maintain a specific cell environment, which supports essential functional processes.
The double layer of the membrane, which consists of phospholipids , is only permeable “ naturally ” to gases and very small, mostly uncharged (and thus hydrophobic ) molecules . Ions and most biologically active substances are polar, i.e. hydrophilic . For them, the lipid bilayer represents a barrier that can only be overcome by additional transport mechanisms.
All life processes and specific cell functions are dependent on the cell or its compartments being in contact with their environment, communicating. Communication means, among other things, the selective exchange of substances and particles. Therefore, mechanisms must be in place that allow molecules to cross the membrane extremely selectively , e.g. B. Channels and so-called carriers .
Transmembrane transport
In most transport processes, which are difficult to consider separately in complex systems (they depend on what is “happening around them”, for example on an upstream transport process), both concentration and charge gradients act with different weightings, and in part syn ergistisch, partly anta gonistisch.
Easy diffusion
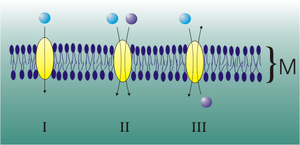
Lipophilic and very small non-polar molecules can pass through the membrane by diffusion . In doing so, they always follow their concentration gradient, trying to balance this out. If the concentration inside and outside the cell is the same, a steady state is established (see Fig. 1, A). In the case of charged particles, the membrane potential also plays a role in establishing the equilibrium.
Passive transportation
Even with passive transport, the molecules cross the membrane without any input of energy from outside or from the cell in the direction of a concentration or potential gradient. Ultimately, passive transport is only a special case of diffusion: even larger molecules and ions, such as sugar , amino acids or nucleotides , for which the membrane is insurmountable, are transported from one side to the other with the help of membrane transport proteins . There are two options here: free diffusion through a plasma membrane and easier diffusion through channel proteins or transport proteins .
Passive transport through channel proteins

The passively transporting channels are transmembrane proteins (also called channel proteins) that span the membrane like a tunnel. They carry polar amino acids towards the inside of the channel . This allows small polar or charged particles such as ions to be transported into the cell through these channels. Different channels have different specificities in terms of conductivity for certain ions or molecules.
Most channels only open when a certain signal is received, which leads to a lock movement, known as "gating". Ligand-controlled channels react to the binding of a messenger substance, for example a hormone . Voltage controlled channels respond to the change in membrane potential . Mechanically controlled channels are regulated by interactions with the cytoskeleton , for example, when the cell shape changes .
Once the channels are open, the molecules diffuse along the concentration gradient across the plasma membrane . This happens either until the concentration of the transported substance is the same on both sides of the membrane, so that the net flow is zero, or until the channels close again (see Fig. 1, B).
Porins have a similar structure to ion channels, but allow significantly larger molecules to pass through. One example are the so-called aquaporins . These form water-conducting channels.
Passive transport through carrier proteins
With passive transport through carrier proteins, the molecule is transported from one side of the membrane to the other by carriers. Carriers specialize in very specific molecules for which they - like enzymes - have a binding site. When the carrier bonds with the substrate, it changes its conformation . As a result of this rearrangement, the molecule in question is channeled through the membrane and released on the other side (see: Fig. 1, E). Every substance to be transported is dependent on its corresponding carrier protein. While some carriers can only carry one molecule at a time ( Uniport ), others have binding sites for two different molecules. They only change their conformation when both binding sites are occupied. The transport occurs for both molecules in the same ( symport ) or in the opposite direction ( antiport ). It should be noted that, in contrast to secondary active transport, there is no dependence on an electrical gradient.
Active transportation

Active transport is defined as a transport process that only takes place in the respective system when energy is supplied from outside. With their help, molecules can be transported against a chemical concentration gradient or ions against an electrical potential gradient.
For the energy balance of the transport of most charged particles, both charge and concentration aspects play a role: Both the reduction of the entropy of a system (build-up / strengthening of a concentration gradient) and the charge transport against the electric field, here the resting membrane potential , require the supply of energy . It should be noted that although energy and charge balances are involved in the system under consideration (here a volume around the transporter), the particle concentrations and their changes due to the semi- / selectively permeable cell membrane must be considered separately.
This energy is essentially made available in 3 ways (often also through a combination of these):
- Chemical binding energy, a typical example is the hydrolysis of ATP ;
- Reduction of a charge gradient as a "driving force", ie electrical energy;
- Increase in entropy in a communicating system, e.g. B. the breakdown of another concentration gradient.
A transport process that takes place against the electrical gradient in the balance is called electrogenic (vs. electroneutral ). With regard to the origin of the energy and the type of work performed, a distinction is made between primary , secondary and tertiary active transport as well as the special case of group translocation .
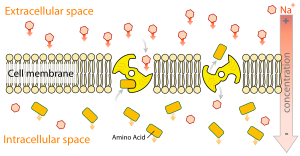
- During primarily active transport , protons and inorganic ions are pumped through the cytoplasmic membrane and out of the cell by transport ATPases while consuming ATP . The H + -ATPase works in plant cells e.g. B. as a proton pump. An ion is pumped from the side with the lower concentration to the side with the higher concentration by a so-called ion pump (Fig. 1 E). The energy comes from the hydrolysis of ATP to ADP and inorganic phosphate (see: Fig. 1, D). An important application for the primarily active transport is the sodium-potassium pump , a protein integrated in the cell membrane which, while consuming ATP, pumps three positively charged sodium ions out of the cell and two positively charged potassium ions in the same cycle -Ions are pumped into the cell. This maintains the resting potential in nerve cells (neurons), which is necessary for the generation and transmission of action potentials .
- The secondary active transport transports a particle (mostly an ion) passively along an electrochemical gradient and uses the potential energy of this gradient to transport a second substrate against its concentration gradient or electrical gradient. If this transport takes place in the same direction, a symport is spoken of as a symporter (e.g. sodium-glucose symport in the small intestine , sodium-iodide symporter in the thyroid ). A transport in the opposite direction is called an antiport via an antiporter , e.g. B. the sodium-calcium antiport through the sodium-calcium exchanger . (See: Fig. 1, C).
- In the case of tertiary active transport , the concentration gradient that a secondary active transport has built up on the basis of a primarily active transport is used. This form of active transport are in the small intestine for. B. Di- and tripeptides taken up by the peptide transporter 1 .
- In group translocation , mostly monosaccharides such as glucose and mannose or sugar alcohols such as glucitol or mannitol are passed through a membrane, whereby the substance to be transported is chemically changed ( generally phosphorylated ) and thus no concentration gradient is created. The best-studied group translocation system is the so-called PEP-PTS ( phosphoenolpyruvic acid phosphotransferase system ) in E. coli . The necessary energy comes from PEP ( phosphoenolpyruvic acid ) instead of ATP . This form of active transport has so far only been found in bacteria .
Diaphragm-displacing transport
Endocytosis
Endocytosis is the invagination process of the biomembrane , in which a single cell or a compartment, a drop of liquid, certain substances dissolved in it, macromolecules or larger food particles up to smaller other cells are incorporated. At the end of the invagination process, a so-called endosome is pinched off or pushed off into the cytoplasm and is now part of the endomembrane system . In this way, the cell absorbs part of the medium surrounding it into its interior (see: Fig. 1, F).
There are four different forms of endocytosis, clathrin-mediated endocytosis , endocytosis via caveolae , phagocytosis and pinocytosis .
Furthermore, the receptor-mediated (or receptor-controlled) endocytosis via clathrin is important, in which special receptors ( asialoglycoprotein receptors ) on the cell membrane surface are responsible for the recognition of the particles to be ingested. For example, LDL particles carry apolipoprotein B-100 on their surface, which binds to the LDL receptor of the cell and thus triggers the uptake of the particle. In this way, for example, cholesterol is absorbed into the cell. After binding to the receptor, due to protein-lipid interactions, the cell membrane turns inside out and forms a coated pit , a depression that is lined with the protein clathrin . The dynamin protein accumulates on the neck of the vesicle that grows in the process. This recognizes with its pleckstrin homologous domain ( pleckstrin homology domain , PH) specifically the phosphoinositol from the membrane. Ampiphysin helps with the arrangement of a dynamin supramolecule, which with its SH3 domain binds the proline-rich domain (PRD) of dynamin and thereby recruits additional dynamin molecules. In the GTP-bound state, the supramolecule lies around the neck of the vesicle as a right-hand helix. During the interaction of the GED and GTPase domains of Dynamin, GTP is hydrolyzed and the Dynamin supramolecule undergoes a conformational change. In the “poppase” theory, this is an increase in the pitch of the dynamin helix, which causes the vesicle to be repelled from the membrane. In the “pinchase” theory, it is this change in conformation that leads to a reduction in the helix diameter and thus to the constriction of the vesicle sac.
Exocytosis
The exocytosis is a process that will be delivered to the cell environment in which substances from the cell. These substances can either be formed in the cell or be indigestible residues from cell digestion. In principle, a transport vesicle ( exosome ) always fuses with the cell membrane during exocytosis (see: Fig. 1, G). The exosome has a simple lipid bilayer (biomembrane) as the outer covering, of which the cell membrane also consists. Most exocytoses are associated with endocytosis (exocytosis-coupled endocytosis). This is necessary to prevent the cell membrane from enlarging unhindered. On the other hand, the cell saves itself the new synthesis of transport vesicles and the associated membrane proteins . This process is known as vesicle recycling .
Transcytosis
Transcytosis (= cytopempsis) is a receptor-dependent transport of extracellular material through the cell and thus a combination of endocytosis and exocytosis. The vesicle is passed on to a neighboring cell or transported into the extracellular space without its content being changed. It occurs in the epithelial cells of the vessels and in the epithelial cells of the intestine, as the spaces between them are blocked by tight junctions .
An example of a receptor for transcytosis is a group of Fc receptors. They are located in the placenta and on the apical side of the child's intestinal epithelium and, through transcytosis, transport maternal IgG to the fetus or the infant.
See also
literature
- Bruce Alberts: Textbook of Molecular Cell Biology. Wiley-VCH, April 2005, ISBN 3-527-31160-2 .
- Helmut Plattner, Joachim Hentschel: Cell Biology. Thieme, Stuttgart January 2002, ISBN 3-13-106512-5 .
- Gerald Karp: Molecular Cell Biology. Springer, Berlin March 2005, ISBN 3-540-23857-3 .
- MA Schlager, CC Hoogenraad: Basic mechanisms for recognition and transport of synaptic cargos. (Review). In: Molecular brain. Volume 2, 2009, p. 25, ISSN 1756-6606 . doi: 10.1186 / 1756-6606-2-25 . PMID 19653898 . PMC 2732917 (free full text).