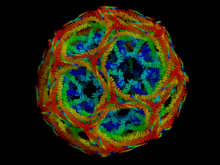
Clathrin
Clathrin is a protein that is involved in the invagination of cell membranes and the formation of vesicles (especially in clathrin-mediated endocytosis ). After the constriction, the clathrin of the spiked vesicles ( clathrin-coated vesicles ) is removed depending on the ATP ("uncoating" by the uncoating-ATPase).
Clathrin is an exceptionally structured protein. It is a hexamer made up of three heavy and three light subunits, each of which has two isoforms . The sub-units are arranged in the form of a tripod, in the form of triskelions . It is thus possible to form a two-dimensional network made up of hexagons and pentagons . No flat plane is created, but a construct with a convex and a concave side. The concave side is always facing the membrane.
The clathrin vesicles have a diameter of 50 to 100 nm and are covered by a framework of mostly 12 pentagons and 8 hexagons from a total of 36 triple skeletons of clathrin. This fibrous protein consists of a heavy chain (180,000 Daltons [Da]) and a light chain (35,000 to 40,000 Da). In addition, the 900 amino acid long dynamin , which can bind and hydrolyze GTP , also occurs in the vesicle coat. Between the clathrin and the outer vesicle membrane, so-called find Adaptorproteinkomplexe (engl. Adapter protein complexes ), abbreviated AP complexes. These complexes, AP-1 to AP-4 (≈340,000 Da), each consist of four different polypeptides, and are therefore heterotetramers. They bind to the heavy chains of the clathrin, membrane lipids and membrane proteins and thereby mediate clathrin binding to membranes. AP-2 mediates the formation of clathrin-coated vesicles (CCV) on the plasma membrane. AP-1 mediates CCV formation on the trans-Golgi network , the last compartment of the protein synthesis apparatus in the secretory transport route.
Clathrin and membrane curvature
Basic textbooks in cell biology name clathrin as the protein that is responsible for the curvature of the membrane during vesicle formation. It was originally assumed that the assembly of the clathrin proteins into the triskelion structure "forces" the membrane into the bent state. However, more recent findings show that other factors play an important role in the effective curvature of the membrane in vivo . This includes B. the activity of ATP-dependent flippases, which produce a locally increased asymmetry of the phospholipid composition in the membrane. The intrinsic property of certain phospholipids to bend membranes then leads to protuberance. The small GTPase Arf involved in the formation of the shell also already has an influence on the membrane curvature. It is therefore the interplay of several complex factors that enable the effective formation of clathrin-coated vesicles .
Web links
literature
- Todd R. Graham, Michael M. Kozlov: Interplay of proteins and lipids in generating membrane curvature. In: Current Opinion in Cell Biology. 22, 2010, pp. 430-436, doi: 10.1016 / j.ceb.2010.05.002 .
- E. Cocucci, F. Aguet, S. Boulant, T. Kirchhausen: The first five seconds in the life of a clathrin-coated pit. In: Cell. Volume 150, Number 3, August 2012, pp. 495-507. ISSN 1097-4172 . doi: 10.1016 / j.cell.2012.05.047 . PMID 22863004 . PMC 3413093 (free full text).