Mannitol
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
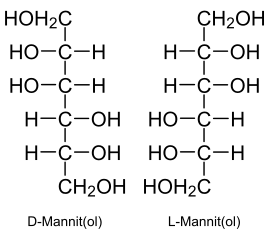
|
|||||||||||||
| General | |||||||||||||
| Surname | Mannitol | ||||||||||||
| other names | |||||||||||||
| Molecular formula | C 6 H 14 O 6 | ||||||||||||
| Brief description |
colorless, sweet-tasting crystals |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| Drug information | |||||||||||||
| ATC code | |||||||||||||
| properties | |||||||||||||
| Molar mass | 182.17 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| density |
1.52 g cm −3 |
||||||||||||
| Melting point |
166-168 ° C |
||||||||||||
| boiling point |
290–295 ° C (4 h Pa ) |
||||||||||||
| pK s value |
13.5 |
||||||||||||
| solubility |
good in water (216 g l −1 at 25 ° C) |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| Toxicological data | |||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Mannitol ( the mannitol), also ( the ) mannitol , is a sugar alcohol and is structurally derived from mannose . It occurs naturally as D- mannitol in salt plants ( halophytes ), but also in mushrooms , algae and animals.
The name comes from manna , and specifically from the sweet sap of the manna ash ( Fraxinus ornus L.). The dried sap of the manna ash contains 13% mannitol.
Mannitol is particularly accumulated in brown algae (up to 40% of the dry matter), mushrooms , lichens , olive trees and figwort plants . Well-known plants with high mannitol contents are figs and olive trees . Mannitol is also found in larch sap and in marine algae of the Laminaria genus (the content can be up to 20%).
Mannitol is obtained from fructose through hydrogenation (such as other polyols sorbitol and maltitol). In winemaking , the mannitol mark is considered a wine flaw.
use
Mannitol is used as a sugar substitute ( additive number E 421 ). In relation to sucrose , a 10% solution has a sweetness of 50–69%. Mannitol is also used as a pharmaceutical excipient ( e.g. for making tablets) and as a medicinal substance in the pharmaceutical industry. It is also used as an additive for heroin and other drugs .
Therapeutic use
Mannitol is the most commonly used osmodiuretic . It is not metabolized in the human organism. Mannitol is filtered unchanged through the kidney corpuscles without being reabsorbed in the further course of the renal tubule . As a result, water in the freshly formed urine ( primary urine ) is osmotically retained, which would normally have been reabsorbed. The water excretion is increased. Displayed is mannitol for the prevention of acute renal failure pre-renal causes (for example in blood and fluid losses after surgery, trauma , states of shock, burns ) and for brain and eye pressure lowering and accelerated diuresis in poisoning .
Mannitol is also used as a mild laxative ( laxative used).
A mannitol solution can be used as a contrast agent in preparation for MRI scans of the small intestine. Mannitol solution is also used in computed tomography as a negative contrast medium to contrast the gastrointestinal tract.
A dry powder inhalant is currently being developed to improve the liquefaction of the mucous layer in the bronchi in the case of cystic fibrosis or the respiratory disease COPD . Mannitol partially replaces the function of the salt that is missing due to the chloride secretion disorder and, by increasing the concentration of water-attracting dissolved particles, increases the fluid content of the periciliary fluid film, which is essential for the function of the cilia (cilia) and the removal of the mucous layer lying on it. In addition, mannitol is said to have a beneficial effect on the viscosity of the mucus and stimulate the action of the cilia. Phase 2 studies are ongoing in cystic fibrosis, phase 3 studies in COPD (as of January 2006).
Use in microbiological test procedures
In microbiology, mannitol is often thiocyanate - agar used for. B. Azotobacter or Staphylococcus to enrich. To do this, soil is applied to the nitrogen-free agar (N 2 fixer). Incubation takes place in the dark at 30 ° C. In this way one is able to detect aerobic acid formers ( bromothymol blue experiences a color change from blue to yellow).
Use as an explosive
Mannitol hexanitrate is a rarely used explosive and initial explosive. Due to its sensitivity, it has mainly been replaced by more safe explosives.
Mannitol synthesis from mannose
The industrial production of synthetic resins from mannitol is no longer common . Mannitol is obtained by reducing mannose. For this purpose, zinc is converted into sulfuric acid in the presence of mannose. This produces mannitol and zinc sulfate.
The actual reaction, however, is a reduction in mannose due to the nascent hydrogen produced when zinc is reacted with sulfuric acid :
Commercial preparations
- Medicines: Osmofundin (DE, AT), Osmosteril (DE, AT) and generic preparations.
See also
Web links
Individual evidence
- ↑ Entry on E 421: Mannitol in the European database for food additives, accessed on August 6, 2020.
- ↑ Entry on MANNITOL in the CosIng database of the EU Commission, accessed on August 6, 2020.
- ↑ a b c d Entry on mannitol. In: Römpp Online . Georg Thieme Verlag, accessed on June 20, 2014.
- ↑ a b c Entry on mannitol in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ a b Data sheet D-Mannitol, 97 +% from AlfaAesar, accessed on December 6, 2019 ( PDF )(JavaScript required) .
- ^ Wissenschaft-Online-Lexika: Entry on mannitol in the Lexikon der Biologie , accessed on April 8, 2009.
- ↑ Kurt Rosenplenter, U. Nöhle (Ed.): Handbook sweeteners. 2nd edition, Behr's Verlag, 2007, ISBN 978-3-89947-262-2 , p. 431.
- ↑ Hans-Dieter Belitz , Werner Grosch and Peter Schieberle: Textbook of food chemistry. 6th edition, Springer, Berlin 2008; ISBN 978-3-540-73201-3 , p. 263.
- ↑ Pharmaxis Ltd. (Australia): Bronchitol , accessed March 18, 2013.

