Wine bug
Wine error is a collective term for undesirable perceptions of a wine in terms of taste, smell (off- flavor ) or appearance, which significantly impair or spoil the desired impression.
The task of the sensory examination includes assessing a wine specifically against the background of typically expected characteristic patterns, determining errors, describing defects and assessing deviations. Wine defects hide certain characteristics or replace them perceptibly with others and can be caused by different ingredients depending on the concentration. Their sensory perception is temperature-dependent; at low wine temperatures they are perceived less strongly than at higher temperatures.
Wine defects can arise in various sub-processes of winemaking - starting with the process of grape harvesting, through the mash, possible enrichment, sulphurisation, fermentation, racking, expansion, maturation, bottling and storage. They can already be created in the grapes, occur during vinification before or after fermentation, only occur during storage or be brought into the wine through external materials. If the defects are caused by microorganisms, it is also referred to as wine sickness .
In a roughly simplified way, cloudiness can be distinguished from impairment of smell and taste, which are caused by microbiological metabolism or without this, and thus three types of wine defects can be distinguished:
- Wine cloudiness, limited to typically clear filtered wines, and discoloration
- Odor and taste defects due to microbiological metabolic products
- Bad smell and taste due to another chemical cause
Wine defects do not include sensory defects such as acidity that is too high or too low or color deficiencies in red wine . These are called wine deficiencies and are seen as a sign of poor quality.
Vine diseases are not described in this article (see also article grape variety ).
Wine cloudiness
Cloudiness is one of the most common defects and can be caused by microorganisms ( yeast , mold or bacteria ) or by foreign substances such as filter media, packaging material or cork. Another source are chemical reactions in which proteins, tannins, heavy metal ions or salts are involved. Unfiltered wines are also "cloudy", but not faulty.
Turbidity caused by microorganisms
From a certain number of cells in the wine, yeasts or bacteria can cause turbidity that is visible to the naked eye. Usually, cloudiness occurs after the wine has been bottled. Re-infection greatly increases the number of microorganisms and undesirable secondary fermentation can occur in the bottle. This process should not be confused with the desired bottle fermentation for the production of high quality sparkling wines.
Molds only play a role in must or grape juice . Intensive research and new breeding attempts to find grape varieties that are as resistant to fungus as possible (so-called piwi grapes) for commercial cultivation.
Chemical cloudiness
The pectin contained in the cell wall of the berries can cause the polysaccharide cloud known as calcium pectate cloudiness . The pectin is a vegetable polysaccharide and depending on the grape variety, the degree of ripeness of the berries and the type of must production, it enters the must. The amorphous turbidity occurs with the release of methanol and subsequent reactions with the calcium . This cloudiness can be avoided by a complete degumming of the must or by an enzymatically supported must clarification .
Heavy metal opacities are mainly caused by iron and copper . Both metal ions react quickly and easily with tannins. In the case of cloudiness due to iron ions (French: casse ferrique), a whitish cloudiness is observed in white wines and some red wines, which can also be deposited as a deposit in the container. With other red wines there is a bluish-black cloudiness. The naturally occurring iron in wine oxidizes and then reacts with phosphate and / or tannins . If a threshold value is exceeded, clouding occurs. The reaction with the iron usually occurs when the wine is drawn from one container (fermentation tank or wooden barrel ) into another. The brief contact with oxygen triggers the oxidation. At low iron contents of 8 to 12 mg / l there is usually no turbidity, however, in the last few years there have been more and more observations where even iron contents below 5 mg / l show turbidity. A legal increase in the possible addition of the nutrient salt diammonium hydrogen phosphate to the must is suspected . The nutrient salt serves primarily as a nitrogen and phosphorus source for the yeast.
Odor and taste defects due to microbiological metabolic products
Vinegar notes, vinegar tinge, or volatile acidity
A vinegar tinge or volatile acidity is understood to mean distinctive changes that smell of vinegar and sometimes can be clearly tasted.
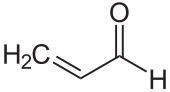
During alcoholic fermentation , yeast breaks down sugar into carbon dioxide and ethanol . This alcohol can then be broken down into vinegar by bacteria. The typical acetic acid tone is created less by the weakly smelling acetic acid than by a series of esterification products such as ethyl acetate . Formic acid is also an essential component of the volatile acid. The influence of propionic acid or fatty acids such as hexanoic acid, on the other hand, is only very insignificant.
During normal alcoholic fermentation, approx. 0.2-0.4 g / l volatile acid is produced. The value can rise to 0.6 g / l in contact with air. These concentrations represent the usual range. The vinegar tinge is caused by additional bacterial transformation. The taste then becomes scratchy and sour and the wine can be cloudy.
Since these reactions can already occur in injured grapes on the vine, it is of great importance to read material that is as ripe as possible without any signs of rot. As a precaution for endangered grapes, the addition of sulphurous acid before the fermentation process applies .
The acetic acid bacteria Gluconobacter oxydans , Acetobacter pasteurianus and Acetobacter aceti are generally held responsible for the off-tone of the volatile acid . On healthy grapes, Gluconobacter oxydans are predominantly found in small numbers of 100 to 1000 per gram of grapes. If, on the other hand, the berries are injured by insects, birds, fungi or hail, an infection with Botrytis cinerea is likely in the early stages (which leads to raw rot in unripe berries ). In this case, the natural yeasts of the berries ferment the fructose into ethanol before harvesting; this promotes the appearance of bacteria such as Acetobacter aceti and Acetobacter pasteurianus . Berries infected in this way can contain up to 1 million acetic acid bacteria per gram of grapes and then give off an intense vinegar smell even in the vineyard.
Solvent clay
A slight smell of solvents (also called “ eagle owl ” in association with the well-known glue ) occurs relatively often in young sweet wines. It is only a real mistake above a certain intensity, although this is much more annoying with red wines.
The solvent clay occurs when acetic acid bacteria settle on damaged grapes and form vinegar. If these grapes are not removed from the grapes, the ethyl acetate, known as a solvent, can form during fermentation together with the alcohol, which is immediately noticeable due to its characteristic odor. Removal is possible - with loss of aroma - using activated carbon .
Mice
A rare mistake is mousing , an unpleasant to disgusting sound that is reminiscent of ammonia and the smell of mouse urine. In the very hot 2003 vintage , the error rate caused by mice increased by leaps and bounds. The problem can arise when the wines have low acid levels or when there is insufficient active sulphurisation.
The cause has not been fully researched; in any case, heterocyclic nitrogen compounds such as acetylpyrroline (ACPY) are sensory . These compounds can be formed by various microorganisms, including yeasts such as Brettanomyces , but also (more rarely) by the occurrence of Lactobacillus species Lactobacillus brevis , Lactobacillus fermentum and Lactobacillus hilgardii . The precursors of the aromatic nitrogen compounds are lysines or ornithine , which are found in every wine. The mousing is currently mainly attributed to 2 isomers of 2-acetyltetrahydropyridine, but other substances also contribute to the false tone:
- 2-acetyl-3,4,5,6-tetrahydropyridine
- 2-acetyl-1,4,5,6-tetrahydropyridine
- 2-ethyl tetrahydropyridine
- 2-acetyl-1-pyrroles .
The formation of these substances requires the presence of alcohol. Therefore, the must is not affected by this off-color.
The high pH values and the high temperatures of the 2003 vintage obviously caused a strong increase in the number of microorganisms that raised the proportion of ACPY above the odor threshold.
The latest research suggests that neither bacteria nor ethanol need to be present for the mouse to develop. The odor-active substances can therefore also arise from methylglyoxal and proline in the course of a Maillard reaction . Corresponding studies were published by Hugo Schanderl as early as 1948 , but received little attention.
Muff and mold tones
Muff rare mold tones come in various degrees of severity are relatively common, they are sometimes referred to as "creeping cork". The sensory perception of "wet cardboard" is characteristic of the Muffton. There are two causes for dull, musty sounds. On the one hand, these unpleasant impressions can come from barrels that are not clean, but this problem is rarely found in the cellar technology because large wooden barrels are not used. The second reason is a typical, common cork bug. The musty tones have the negative characteristic of slowly getting stronger after opening a faulty bottle.
Sulfur or Böckser
There are several different wine defects caused by sulfur compounds.
In young wines that are bottled fresh, is the aftermath of the sulfurization free sulfur dioxide occur. A pungent, unpleasant smell in the glass is typical; in extreme cases, the eyes can water easily. This pungent sound should evaporate after minutes in the air.
Worse are the different versions of the Böckers (sometimes also written Böxer ). The yeast can convert sulphites , which are introduced into the wine through sulphurisation, into hydrogen sulphide , a substance with a typical odor of rotten eggs. This variant is mainly found in young wines. If this is not recognized in time or even overlooked, a so-called "bearing blocker" can develop. Various secondary reactions (some of which have not yet been fully clarified) lead to the formation of more complex sulfur compounds, in particular ethyl mercaptan . There are nuances of smell reminiscent of the "rotten eggs" already mentioned, others reminiscent of burnt rubber, garlic, onions or boiled cabbage.
As a preventive measure against Böckser, the sediments of the must should be removed. The wine error can be corrected within limits by adding copper compounds or silver chloride .
Finally, sulfuric acid varnish occurs when too little sulfur is added during the aging of the wine. This creates a taste and smell reminiscent of sherry. Sulphurisation is also possible and then leads to an unpleasantly sour taste.
Atypical aging tone, UTA
The atypical aging tone , UTA for short , has only been described for a relatively short time. White wines appear dull, as if excessively aged, reminiscent of vomit . UTA is also called a mothball or naphthalene note . Other synonyms for the UTA are acacia , soap , detergent clay , nitrogen gas and dirty-wet laundry clay . The taste of such wines is described as dull and expressionless, the wine color as pale to water-white.
The 2-aminoacetophenone (AAP) produced by yeasts was identified as the trigger for the UTA , which is already perceived in very small quantities of <1 µg / l. Since there is a connection between UTA and weather influences, it is generally the case that in hot, dry years the stress on the plant during the ripening period promotes the development of UTA. It is assumed that this is due to a lack of nitrogen supply due to the drought and water stress in the ripening phase due to global warming .
Bitter tone or acrolein tinge
The bitter tone (English: bitterness taint ; French: amertume ) is one of the rare mistakes. The smell of propenal (also commonly called acrolein) is caused by bacterial degradation of the glycerine in wine. Propenal itself is not bitter, but can result in a bitter taste if it reacts with anthocyanins . These plant pigments are mainly found in the peel of the berries, so that mostly red wines are affected by this type of defect. The limit of perception is around 10 mg / l. The lactic acid bacteria Pediococcus parvulus and Lactobacillus cellobiosus transform glycerine into 3-hydroxyproprionaldehyde with the help of the enzyme glycerine dehydratase. A further dehydration of this intermediate product during aging ultimately results in propenal.
Butter clay or whey ketone
Diacetyl is the simplest member of the diketone class . It has a distinctive taste and smell of butter and is also part of the natural butter aroma. In small quantities, the substance even supports the aroma of the wine and gives it a slightly nutty or caramel-like note and values of approx. 0.3 mg / l are absolutely normal. However, concentrations of over 5 mg / l are already perceived as unpleasant. The metabolic pathway for the formation of diacetyl by means of lactic acid bacteria has not yet been fully clarified.
Geranium tone
An unpleasant sound reminiscent of the smell of geraniums (pelargoniums), which is primarily associated with the substance geraniol .
The geranium is created by the breakdown of sorbic acid by bacteria. It can only arise in wines that have been stabilized with sorbic acid as a preservative. In the production of wine, the addition of a maximum of 200 mg / l (Germany and Austria) or 1 g / l (USA) to the must or wine is permitted, as this addition protects the wine from yeast and mold. However, since sorbic acid does not act against acetic and lactic acid bacteria and is reduced by these to sorbinol ( E, E -2,4-hexadien-1-ol), undesirable and irreversible changes in taste can occur. Subsequent etherification with the ethanol from the wine forms 2-ethoxy-3,5-hexadiene . This is already perceptible in very low concentrations (typically 0.1 µg / l).
Mannit stitch
Mannitol or mannitol is a sugar alcohol that is created by breaking down sugar ( redox reaction of fructose ) by means of heterofermentative lactic acid bacteria. As the cause are Leuconostoc dectranicum , Lactobacillus pentoaceticus and Lactobacillus brevis . The sweet mannitol is usually produced in wine during the malolactic fermentation when the residual sugar content is still too high. Since acetic acid and 2-butanol are also formed, this wine error is expressed in terms of smell by an ester tone , combined with a sweet, scratchy finish .
Toughening
The toughening or Lindwerden ( Engl. Ropiness , ropy, slimy; double. Graisse ) is a viscosity increase by the formation of polysaccharides in the form of dextran from the residual sugar. Streptococcus mucilaginosus var. Vini and Pediococcus damnosus are responsible for the formation of the colloids . However, the wine is neither analytically nor sensory changed.
Petrol tone
On petroleum , kerosene reminiscent smell or taste; difficult to distinguish as an error.
Petrol tint occurs relatively frequently and is caused by 1,1,6-trimethyl-1,2-dihydronaphthalene, can be a mistake in young wines, but is typical of the variety in some older wines (such as ripe Rieslings).
The sensitivity for petrol tones varies greatly from region to region, on the other hand the various grape varieties show great differences in their aging behavior. Typically, Rieslings , but also Traminer, sometimes develop strong petroleum notes when stored for a long time. These taste impressions are atypical or undesirable and flawed, at least in younger wines.
Often certain terroir notes (e.g. slate as a base) are confused with petrol.
Brettanomyces
The trigger is the yeast Brettanomyces (Dekkera) bruxellensis , which can lead to another animal aging note that is not typical for wine. The false tone, often referred to in the literature as "Brett" or "Brett-Fehlton" after its yeast strain, is referred to as "stable odor", sometimes as "horse sweat," "horse saddle" or "wet dog" and describes weaker or stronger perceptions , complex animal notes. The sensory perception ranges from sweetish sharp notes to leathery, smoky, tar-like, sour or medicinal and pharmaceutical-like notes. The taste -forming substances are 4-ethylphenol , 4-ethylguaiacol and 4-ethylcatechol , whereby the individual limit of perception is different.
Earthy tone
Geosmin is a naturally occurring bicyclic alcohol . The substance has a pronounced earthy, musty smell and taste and is partly responsible for the sensation perceived as a typical floor smell, but also for the smell of mold . Geosmin is also involved in the odor perception that occurs when it rains, especially after a long period of drought.
The human sense of smell is highly sensitive to geosmin; the odor threshold is 10 −10 . The substance is produced by certain strains of the Penicillium in the presence of Botrytis cinerea .
Aldehyde clay
If the alcohol contained in the wine is further oxidized enzymatically , ethanal , better known under the common name acetaldehyde, is formed .
This aldehyde has an idiosyncratic, strangely fruity, dull, rather unpleasant smell and taste that is difficult to describe. This error usually arises when the wine has not received sufficient sulfur in the form of free SO 2 .
Cork clay
2,4,6-Trichloroanisole (TCA) causes the best known and most common wine defect, the cork tone , which is also known regionally as a stopper . How exactly the trichloro anisole enters the bark of the cork oak or subsequently into the product made from it, the cork , has not yet been fully researched. The most common cause is probably bleach in cork processing, but also in storage boxes. In addition, ingredients of wood preservatives or their breakdown products come into question. Likewise, cleaning agents containing chlorine in the wine cellar can be partly responsible. Therefore, a cork tone can occur, albeit rarely, not only in bottles with natural corks, but also in those with screw caps and other alternative closures. Trichloroanisole is one of the phenol derivatives and, like many phenol derivatives, is harmful to health.
TCA has a low odor threshold in wine and can be perceived by experienced wine testers from a concentration of 0.001 µg / l (1 nanogram) in white wines and from 0.005 µg / l (5 nanograms) in red wines. The cork clay is characterized by a typical corky smell and taste; in certain concentrations it can also take on leathery, musty tones. In addition, there are errors that are referred to as "creeping cork", including not clearly definable, dull, sometimes slightly musty notes that can often only be recognized in direct comparison with intact bottles. Cork defects can become significantly more pronounced in the air; when mixing a corky wine with mineral water, the carbonic acid significantly increases the perception of the defect.
literature
- Reinhard Eder u. a .: Wine fault. Österreichischer Agrarverlag, Leopoldsdorf 2003, ISBN 3-7040-1957-7 .
Web links
Individual evidence
- ↑ Eva Derndorfer: Wine Sensory. From science to practice. AV-Verlag, Vienna 2009, p. 112.
- ↑ a b duToit, M., Pretorius, IS (2000). “Microbial spoilage and preservation of wine: Using weapons from nature's own arsenal - A review”. South African Journal of Enology and Viticulture 21: pp. 74-96.
- ↑ Marais, Johann Flavorful nitrogen containing wine constituents ( Memento of September 28, 2006 in the Internet Archive ). Wynboer. Last access to this page on February 10, 2008.
- ↑ L. Künzler, M. Pour Nikfardjam, New Findings on the Origin of the Mouse Tone in Wine, Deutsches Weinbau-Jahrbuch 2014, pp. 170–177.
- ↑ Reinhard Eder u. a .: Wine fault. Österreichischer Agrarverlag, Leopoldsdorf 2003, pp. 101–102.
- ↑ Rapp A. u. a .: 2-Aminoacetophenone: causative component of the "atypical aging note" ("naphthalene clay", "hybrid clay") in Wein , Vitis 32, 1993, pp. 61-62.
- ↑ a b Reinhard Eder u. a .: Wine fault. Österreichischer Agrarverlag, Leopoldsdorf 2003, pp. 59–60.
- ↑ T. Hühn et al .: Microorganisms in winemaking in microorganisms in winemaking ( Memento from January 12, 2012 in the Internet Archive ), COMMUNICATIONS FOR SCIENCE AND TECHNOLOGY, CDR 3 edition, November 1999, pp. 42–88; P. 69.
- ↑ T. Hühn et al .: Microorganisms in winemaking in microorganisms in winemaking ( Memento from January 12, 2012 in the Internet Archive ), COMMUNICATIONS FOR SCIENCE AND TECHNOLOGY, CDR 3 edition, November 1999, pp. 42–88.
- ↑ Eva Derndorfer: Wine Sensory. From science to practice. AV-Verlag, Vienna 2009, pp. 111–112.
- ↑ Eva Derndorfer: Wine Sensory. From science to practice. AV-Verlag, Vienna 2009, pp. 110–111.