Sulfites
Sulphites are the salts and esters of the sulphurous acid H 2 SO 3 . The salts contain the sulfite ion (SO 3 2− ) as an anion . They are widely used as preservatives in wine , dried fruit, and potato products. However, sulphites also occur naturally in almost all wines.
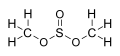
The sulphurous acid esters with the general formula ROS (= O) -OR '(with R and R' as organic radicals ) are also referred to as sulfites .
Sulphurous acid is a biprotonic acid. Hence, among the salts, there exist
- Sulphites (M I 2 SO 3 ), which are also called normal, neutral or secondary sulphites and
- Hydrogen sulfites (M I HSO 3 ), which are also called primary or acid sulfites or bisulfites .
Hydrogen sulfites do not exist as solid salts and are only present in aqueous solutions. When a solution is concentrated, hydrogen sulfites react to form disulfites (S 2 O 5 2− ), splitting off water and forming a sulfur-sulfur bond :
Under acidic conditions, sulphites and hydrogen sulphites release sulfur dioxide :
use
Sulphites are used as reducing agents. The hydrogen sulfite anion reacts in chemical reactions as a nucleophile (e.g. with aldehydes to form salts that crystallize well ). Important processes for the production of pulp and paper from wood work with sulfites, see sulfite process (including calcium hydrogen sulfite , according to Mitscherlich ).
Sulphites in wine
The labeling “contains sulphites” or “contains sulfur dioxide ” is mandatory according to Art. 3 Para. 3 of the Wine Market Organization Implementation Ordinance for concentrations of more than 10 mg / l. In the United States , wines bottled after mid-1987 must include a reference to sulfites on the label . The corresponding regulation in the EU has been in force since 2005. The labeling requirement goes back to the fact that people who are hypersensitive to sulfites have intolerance reactions when consuming even small amounts of sulfite. B. bronchospasm and asthma , anaphylactoid reactions, urticaria and low blood pressure .
Sulphites are produced naturally in small quantities (10–30 mg / l) during the alcoholic fermentation of the wine. The antimicrobial and antioxidant effects of sulfur have been known since the end of the 18th century . Since then, the addition of sulfur has been firmly anchored in global wine production. Amounts of sulfur dioxide between 90 and 400 mg / l are used in wine. Sulfur dioxide (SO 2 ) is added to wine in gaseous form, in an aqueous solution, as "sulfur powder" ( potassium disulfite ), in the form of tablets or, as in the past, by burning out barrels with sulfur shavings.
Sulphites make it possible to store wines for a longer period of time without the wines completely " overturning " due to oxidation , which means that enjoyment is only limited or impossible. In addition, they prevent unwanted secondary fermentation in the bottled bottle of residual sweet wines, as they effectively prevent microorganisms (such as yeast ) from doing their work.
The addition of sulphites is also permitted in organically grown wines and must also be labeled on the bottle.
In some places there are efforts within the wine industry to produce wines without the addition of sulfur dioxide. A few conventional as well as organic wineries have been successfully doing this for a number of years, which is mainly thanks to modern wine technology .
For sulfur dioxide in wine, there are maximum limits according to EC-VO.
| Type of wine | EC maximum limit SO 2 total |
|---|---|
| Red wine <5 g / l residual sugar | 150 mg / l (until July 31, 2009: 160 mg / l) |
| Red wine > 5 g / l residual sugar | 200 mg / l (until July 31, 2009: 210 mg / l) |
| White wine & rosé wine <5 g / l residual sugar | 200 mg / l (until July 31, 2009: 210 mg / l) |
| White wine & rosé wine> 5 g / l residual sugar | 250 mg / l (until July 31, 2009: 260 mg / l) |
| Late harvest and comparable foreign wines | 300 mg / l |
| Selection and comparable foreign wines | 350 mg / l |
| Beerenauslese and Trockenbeerenauslese , ice wine and comparable foreign wines | 400 mg / l |
| Wines with the note "suitable for diabetics" (no longer allowed since July 1st 2007) | 150 mg / l |
DNA methylation
Bisulfites can selectively react with cytosines in DNA . This is used in bisulfite sequencing to determine methylated DNA .
proof
The qualitative proof can be done indirectly with permanganates . These discolor in a redox reaction when sulfites are present.
- Sulphite ions react with permanganate ions in an acidic environment to form manganese (II) ions, sulphate ions and water.
The reaction is not specific for sulfites and can therefore only be used as evidence for sulfites if the presence of other reducing agents is excluded. Likewise, an iodine solution is decolorized by sulfites, whereby iodine is reduced to iodide and sulfite is oxidized to sulfate .
With sodium nitroprusside in the presence of zinc ions, a red precipitate of Zn 2 [Fe (CN) 5 SO 3 ] is formed. With barium chloride solution, a white precipitate of barium sulfite forms , which, unlike barium sulfate, is easily soluble in acids.
Examples
Are salts of sulphurous acid
- Potassium sulfite
- Sodium sulfite
- Magnesium sulfite
- Sodium bisulfite (sodium bisulfite)
- Calcium hydrogen sulfite (calcium bisulfite).
Are esters of sulphurous acid
See also
literature
- Pascal Ribéreau-Gayon, Denis Dubourdieu, Bernard Donèche, Aline Lonvaud u. a .: Traité d'oenologie, 1. Microbiologie du vin, Vinifications . Dundo, Paris 2004, ISBN 2-10-007301-X .
Web links
Individual evidence
- ↑ a b Commission Regulation (EC) 753/2002 of April 29, 2002, full text
- ↑ Federal Institute for Risk Assessment : Very high levels of sulfur dioxide in wine. (PDF; 108 kB) November 13, 2003.
- ↑ Hans Peter Latscha, Gerald W. Linti, Helmut Alfons Klein: Analytische Chemie , Springer, Berlin, 4th ed., 2004, p. 52 Google Books
- ↑ Jander-Blasius: Textbook of analytical and preparative inorganic chemistry , 5th edition, S. Hirzel, Stuttgart-Leipzig 1965, pp. 132-133.

![{\ mathrm {2 \ HSO_ {3} ^ {-} \ \ rightleftharpoons \ [O_ {2} S {-} SO_ {3}] ^ {{2 -}} + H_ {2} O}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/36494f459b9058c12aa7344a18ea3ce5d8c7cb5e)