Sodium hydrogen sulfite
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
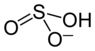
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Sodium hydrogen sulfite | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | NaHSO 3 | |||||||||||||||||||||
| Brief description |
colorless to yellowish liquid (only exists in solution) |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 104.06 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| MAK |
Switzerland: 5 mg m −3 (measured as inhalable dust ) |
|||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Sodium bisulfite (NaHSO 3 ), and sodium bisulfite, is a sodium - salt of sulfurous acid . It is not stable outside of aqueous solutions.
presentation
An aqueous solution of sodium disulfite gives sodium bisulfite.
Passing sulfur dioxide through a solution of sodium carbonate also produces sodium hydrogen sulfite.
properties
It is commercially available as a solution with a sodium hydrogen sulfite content of between 37 and 50%. These solutions have caustic , reducing and strongly bleaching properties. When the aqueous solution is concentrated, sodium disulfite (Na 2 S 2 O 5 ) or sodium sulfite is formed .
The addition reaction with aldehydes or ketones to form so-called hydrogen sulfite adducts ( bisulfite adducts ) is important, the sulfur of the hydrogen sulfite ion adding nucleophilically to the carbonyl group to form a hydroxyalkyl sulfonate ion. As an example, the reaction with formaldehyde to form the sparingly soluble sodium hydroxymethyl sulfonate is demonstrated here:
The hydrogen sulfite addition is often used to purify aldehydes or ketones, since it is reversible under basic or acidic conditions.
use
As a food additive E 222, sodium hydrogen sulfite is used as a preservative e.g. B. in starch and sugar production , antioxidants and as a stabilizer in food as well as for the analysis of methylation patterns in DNA. It is also used as a reducing agent in the chemical industry for wastewater treatment. It is also used in the paper industry . It is used in sulfomethylation to manufacture detergent substances , ion exchangers and water-soluble resins.
Individual evidence
- ↑ Entry on SODIUM BISULFITE in the CosIng database of the EU Commission, accessed on April 17, 2020.
- ↑ a b c Entry on sodium hydrogen sulfite, aqueous solution in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ Entry on sodium hydrogen sulfite in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limits - Current MAK and BAT values (search for 7631-90-5 or sodium hydrogen sulfite ), accessed on November 2, 2015.
- ↑ Sodium hydrogen sulfite data sheet (PDF) from Merck , accessed on July 23, 2008.
- ↑ Beyer-Walter: Textbook of Organic Chemistry , 23rd Edition, S. Hirzel Verlag, Stuttgart 1998.
- ↑ Grillo-Werke AG | SODIUM BISULPHITE LIQUID . ( grillo.de [accessed November 8, 2018]).
- ↑ CM Suter, RK Bair, FG Bordwell: The sulfomethylation reaction. In: Journal of Organic Chemistry , 10.5, 1945, pp. 470-478.




